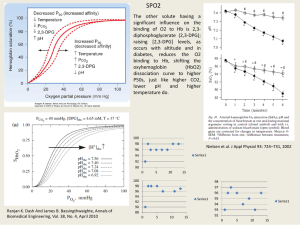
Observations on histological methods involving the use of phosphotungstic
реклама

311 Observations on histological methods involving the use of phosphotungstic and phosphomolybdic acids, with particular reference to staining with phosphotungstic acid / haematoxylin By D. BULMER (From the Anatomy Department, The University, Manchester 13) Summary An attempt has been made to elucidate some of the factors involved in differential staining of tissues with Mallory's phosphotungstic acid / haematoxylin, and to find out how far these factors are applicable to other histological methods in which phosphotungstic or phosphomolybdic acid is used. Sections have been subjected to various pretreatments to find whether specific chemical groupings are responsible for any of the staining reactions, but the results obtained are not always easy to interpret. The most important single factor in determining the staining reaction of a tissue material with PTAH appears to be its relative permeability to the two colour-complexes of the PTAH mixture. The red complex, of larger molecular size, penetrates collagen; muscle-fibres are penetrated mainly by the smaller blue complex, while red bloodcorpuscles fixed with formaldehyde are not penetrated by either. The staining reactions of muscle and red blood-corpuscles can be altered by a methylation procedure, by treatment with performic acid or formic acid, or by mild alkaline hydrolysis ; but this appears to be due to alteration of permeability rather than to chemical alteration of any specific dye-binding groups. The effects of blocking reactions indicate that the binding of both complexes is to basic groups in the tissues, though it is possible that hydroxyl groups and carboxyl groups may also be involved. It appears that similar factors control the differential staining with other techniques which involve the use of complex acids (Baker, 1958), and that the chemical specificities which have been claimed for some of them are not well founded. Introduction D E S P I T E the extensive use of phosphotungstic and phosphomolybdic acids in histological practice there has been little agreement on the mechanism of the several methods in which they are employed. Baker (1958), referring to the publications of earlier workers, considered that differential staining with acid dyes, as in trichrome methods, is dependent upon differences in the permeability of proteins to dye molecules of differing molecular sizes, but other recent workers appear to have largely ignored this viewpoint. Thus, Monne and Slautterback (1951), using the Azan technique on sea-urchin eggs, suggested that the aniline blue was bound by the amino-sugars of mucopolysaccharides and the azocarmine and orange G by the amino-groups of proteins. Hrsel (1957) introduced a staining technique employing treatment with phosphomolybdic acid, eosin, and light green, after previous mordanting of the section with chromic acid. From the use of blocking techniques he deduced that the light green stained protein-bound amino-groups while the [Quart. J. micr. Sci., Vol. 103, pt. 3, pp. 311-23, 1962.] 312 Buhner—Phosphotungstic acid j haematoxylin eosin was specific for proteins containing tryptophane. Jones (i960), however, made an observation which appears to support Baker's view. He found that tanned proteins stained with the orange G of Mallory's phosphotungstic acid / aniline blue / orange G mixture. After destruction of the tanning bonds with diaphanol the orange G stainability was replaced by staining with the aniline blue, but this process could be reversed by re-tanning the protein with quinone. Landing, Uzman, and Whipple (1952) described the use of phosphomolybdic acid in a histochemical method for the demonstration of choline. They believed that the complex acid was bound to choline residues in the tissues, and could subsequently be demonstrated in situ by reduction to molybdenum blue. Treatment of formalin-fixed frozen sections with various reagents increased the number of structures binding phosphomolybdic acid, and Landing and his colleagues supposed that this was due to the freeing of bound choline, which had previously been unable to react. Pearse (i960), quoting the method, comments on the increased number of reacting sites in paraffin sections compared with frozen sections, but does not appear to accept that these are necessarily associated with the presence of choline. The reaction of phosphomolybdic acid with collagen was investigated by Puchtler and Isler (1958). They found that the binding of the complex acid, which they demonstrated by the molybdenum blue reaction, resulted in an intense basiphilia of the collagen. They believed that the phosphomolybdic acid became bound to basic groups of the tissue proteins, leaving several of its own acidic groups free for staining with basic dye, and that in trichrome methods the amphionic aniline blue acts as a basic dye and is held by the free acidic groups of bound complex acid. A related problem which has received little recent attention is that of the so-called metachromatic staining produced by Mallory's phosphotungstic acid / haematoxylin mixture. Tissues which take the fibre stain of a trichrome method are usually red in a phosphotungstic acid / haematoxylin preparation, while those which take the plasma stain of a trichrome method are usually blue. This paper records an attempt to elucidate some of the factors which may be concerned in the differential staining with phosphotungstic acid / haematoxylin and to find how far these are also applicable to other staining methods which employ the complex acids. Methods and results The phosphotungstic acid / haematoxylin mixture (PTAH) contains 2% phosphotungstic acid (PTA) and o-i% haematoxylin. It is ripened either naturally or, as in the present investigation, by the addition of 17-7 mg % of potassium permanganate. Mallory's technique (Mallory and Parker, 1929) prescribes fixation in Zenker's fluid and removal of mercury from the section with alcoholic iodine. The section is then exposed to 0.5% permanganate for 5-10 min and 5% oxalic acid for 10-20 min(reduced by Lillie(i954)to 5 min), stained in the PTAH mixture for several hours, and taken straight to 95 % Buhner—Phosphotungstic acid j haematoxylin 313 alcohol and dehydrated, cleared, and mounted. It has been pointed out by Peers (1941) that formalin-fixed sections produce satisfactory results after preliminary mordanting with mercuric chloride, while Earle suggested (Lillie, 1954) that mercurial treatment could be omitted. Sections of rat uterus were employed as test material and blocks were fixed in 4% neutral formaldehyde or in pure acetone for 24 h. Formalin-fixed sections of rat striated muscle and tendon were also used. Adequate staining was obtained in the formalin-fixed sections without any pretreatment, though smooth muscle tended to have a deep purple rather than a pure blue colour. Nucleoli were stained intensely blue, while the rest of the nuclear material was sometimes blue and sometimes red. This may correspond with the different staining of whole and sectioned nuclei with a modified Azan technique (Lison, 1955). The purple staining reaction of smooth muscle appears to be due to the presence of both blue and red colours, and it is interesting that the blue binds much more slowly than the red. After a few minutes in the staining mixture collagen is stained strongly red and muscle less markedly so—only after about 15 min does the blue stain become more prominent in muscle. Mordanting in a saturated solution of mercuric chloride at 5 8° C for 3 h (Peers, 1941), followed byremoval of the mercury, intensified the blue staining of the muscle-fibres and the staining of collagen, though the latter become orange rather than red. Treatment with permanganate and oxalic acid after the mercurial mordanting, as prescribed by Mallory and Parker (1929), reduced the staining to a level rather less than that obtained in the untreated formalin-fixed sections. Treatment of the formalin-fixed sections with permanganate and oxalic acid, without previous mercurial mordanting, produced weak staining of collagen and patchy, irregular blue staining of muscle. There was no apparent difference in the behaviour of formalin-fixed and acetonefixed sections, except in the staining of red blood-cells. With formalin fixation the majority of the red blood corpuscles were unstained, while in the acetonefixed material they were a deep blue. The PTAH mixture continues to ripen further for several months after the initial artificial oxidation. With an old mixture the staining of formalinfixed sections is intense, with a deep blue colour in the muscle-fibres. Mercurial mordanting has much less effect on the staining of muscle than with a fresh PTAH mixture, and red cells usually stain blue without any pretreatment. In an attempt to elucidate the mechanism of staining, sections were submitted to a variety of pretreatments before exposure to PTAH. For this purpose formalin-fixed sections were usually employed, without mercurial mordanting or permanganate-oxalic acid treatment, and staining was carried out in a PTAH mixture within one month of artificial ripening. Deamination. Treatment with van Slyke's reagent for 24 h before exposure for several hours to PTAH produced slight reduction in the staining of 314 Buhner—Phosphotungstic acid j haematoxylin collagen and rather more marked impairment in the staining of muscle. The muscle was, however, a pure blue, in contrast to the purple of the control. When a nitrosated section was treated for a short period with PTAH (about 30 min) and compared with a control section exposed to PTAH for the same period there was complete abolition of staining in the muscle while the staining of collagen was only slightly impaired. There are various possible explanations of this late appearance of muscle staining after nitrosation. In particular, the effect of nitrous acid on some basic groups may be in part reversible by exposure to the staining mixture, or the groups which still bind the blue stain maybe ones that are resistant to nitrosation but in which staining is normally delayed. Treatment with 0-4% ninhydrin for 8 h at 8o° C (Monne and Slautterback, 1951) effectively abolished the staining of muscle and nuclei with PTAH. The staining of collagen was very much impaired, and of a purplish tinge. These effects, however, were probably not those of a straightforward oxidative deamination, since the interposition of mild alkaline hydrolysis after the ninhydrin treatment produced red staining of both collagen and muscle. It is likely that ninhydrin has a tanning action, as described by Speakman (1955), and that the change in the staining picture after alkaline treatment is due partly to hydrolysis of some of the bonds involved in the tanning, and partly to the usual effect of alkaline pretreatment on staining with PTAH (see below). Benzoylation. Treatment with 10% benzoyl chloride in pyridine for 24 h completely abolished the staining of muscle. The red staining of collagen was very much reduced, while nuclear staining was apparently unaffected. It is interesting that the resistance to benzoylation resembles that occurring in association with the coupled tetrazonium reaction (Pearse, i960). Acetylation. When sections were treated for 24 h with 40% acetic anhydride in pyridine and exposed for the usual period of several hours to PTAH, the staining of muscle-fibres was strong, with a pure blue colour instead of the usual purple shade. The staining of other tissues was unaltered, except for a change in the staining reaction of the basement membrane of the uterine epithelium from red to blue. If, however, the sections were examined after a short period in the staining mixture and compared with untreated controls stained for the same period, it was seen that muscle, nuclei, and basement membrane were unstained in the acetylated sections but quite strongly stained in the controls. The staining of collagen was reduced in the acetylated sections, and its colour purple. It appears that the acetylation is largely reversed by prolonged exposure to the PTAH mixture, though a slight residual effect, producing the bluer staining of muscle, remains. Controls exposed to pyridine alone did not differ in staining from untreated sections exposed to PTAH. In an attempt to reverse the O-acetylation and leave the N-acetylation intact, sections which had been acetylated were exposed to mild alkaline hydrolysis. With short exposure to PTAH they gave normal red staining of collagen, deep blue staining of the basement membrane and red staining of Buhner—Phosphotungstic acid / haematoxylin 315 muscle. Efforts to produce a specific N-acetylation, by methods adapted from those of Green, Ang, and Lam (1953), were unsuccessful. Phosphorylation. Results similar to those of acetylation were produced by phosphorylation with phosphorus oxychloride in pyridine or in chloroform. Sections subsequently exposed for a short time to PTAH gave no staining of muscle or nuclei, while collagen was distinctly blue. More prolonged treatment with PTAH resulted in the normal red staining of collagen with blue staining of muscle. The findings of protein chemists (Herriott, 1947) suggest that the phosphorylation will affect both hydroxyl and amino-groups, and the reversal of phosphorylation by the acidity of the PTAH mixture is not unexpected. Methylation. Sections were treated for 24 h at 60° C with methanol containing o-i% hydrochloric acid before exposure to PTAH. The staining of collagen was unaltered while muscle-fibres and nuclei were red instead of their usual blue. Red blood-corpuscles, which in untreated formalin-fixed sections did not stain, were intensely blue, but the blue staining was converted to red by more prolonged exposure to the methylating procedure. The alteration in the staining picture produced by methylation was incompletely reversed by exposure of the methylated section to 0-5% potassium permanganate for 20 min before staining (Lillie, 1954). Since the permanganate treatment of the usual PTAH technique weakens the staining of muscle, the restoration of the blue staining in a methylated section may possibly be a true demethylating effect. Alkaline hydrolysis. Treatment with a weakly alkaline solution (dilute ammonia or sodium borate solution) before PTAH produced red staining of muscle and intense blue staining of red cells. A similar effect was obtained by treatment with a 6 M solution of urea (pH about 7-5), but the alteration in muscle staining was incomplete. Strong lithium bromide solution had no apparent effect on subsequent staining with PTAH. Performic acid. Performic acid, prepared by the method of Pearse (i960), produced a result similar to that of alkaline hydrolysis, though the alteration in muscle staining was often incomplete. Prolonged exposure to performic acid did not affect the intense blue staining of the red cells, but methylation after the performic acid treatment induced red staining. Significantly, comparison with control sections showed that pure formic acid produced results identical with those of the performic acid mixture. Acid hydrolysis. Exposure to N-hydrochloric acid for 1 h slightly reduced the staining of muscle and collagen, without altering the colour of either. Impairment of nuclear staining was more pronounced. Periodic acid. Exposure to a 2% solution for 1 h had no apparent effect. Lipid extraction. Treatment with hot methanol/chloroform for 48 h did not reduce subsequent staining of any tissue with PTAH, and the deep blue of muscle was rather accentuated. The foregoing results resemble those reported by Hrsel (1957) on the effects 316 Buhner—Phosphotungstic acid j haematoxylin of blocking agents on staining with his technique. Generally, the effects of blocking agents on red staining with PTAH are similar to their effects on light green staining with Hrsel's technique, and the effects on blue staining with PTAH are similar to those on the staining with eosin. Methylation and alkaline hydrolysis were not included in Hrsel's investigations, and periodic acid, which Hrsel found to abolish staining with eosin, has no corresponding effect on staining with PTAH. To extend the comparison further, similar pretreatments to those used with PTAH were employed on sections which were subsequently stained for i h in Mallory's PTA / aniline blue / orange G mixture. The results which were obtained indicate a close correspondence between red staining with PTAH and staining with the aniline blue of Mallory's mixture, and between either blue staining or lack of staining with PTAH and staining with the orange G of Mallory's mixture. Deamination somewhat reduced the staining of collagen with Mallory's mixture—much more with fine than with coarse fibres—while muscle stained a pure orange instead of the rather greyish orange produced in a control. Benzoylation grossly impaired staining with aniline blue, but because of the overall yellow colour induced by the benzoylation procedure the effect on staining with orange G was not clear. Phosphorylation and acetylation considerably reduced the staining of collagen with aniline blue, while muscle was a pure orange. Methylation or alkaline hydrolysis induced strong staining of muscle with aniline blue. An attempt was made to show the binding of phosphotungstic acid to tissue sections by means of the tungsten blue reaction, in a way similar to the use of the molybdenum blue reaction by Landing and his colleagues (1952) and by Puchtler and Isler (1958). This was unsuccessful, because of the low intensity of the tungsten blue. To some extent, however, the bound phosphotungstic acid could be demonstrated indirectly by the increased basiphilia resulting from its binding to the tissues, comparable with the basiphilia associated with the binding of phosphomolybdic acid (PMA) described by Puchtler and Isler. Sections were therefore exposed to 2% PMA for 5 min, and subsequently treated either with acid stannous chloride to produce the molybdenum blue reaction or with a basic thiazine dye to demonstrate the alteration in basiphilia. Sections exposed for 5 min to 2% PTA were treated only with the basic dye. PTA and PMA produced similar changes in basiphilia of various tissue structures, so that it may be justifiable to use the distribution of the molybdenum blue reaction after PMA treatment as an indication of the sites at which PTA will also bind. It must be realized, however, that failure to obtain a molybdenum blue reaction in a tissue after PMA treatment indicates only the absence of bound hexavalent molybdenum (Sidgwick, 1950). PMA treatment may possibly have some effect on a tissue even when there is no subsequent molybdenum blue reaction. The results which were obtained by these methods are shown in table 1. Without previous treatment muscle-fibres bound less PMA than collagen, while red cells bound none. The basiphilia of muscle-fibres after PMA or Buhner—Phosphotungstic acid / haematoxylin 317 PTA treatment was less intense than that of collagen, though staining with basic dye persisted down to very low pH levels. Methylation or alkaline hydrolysis before treatment with complex acid increased the intensity of the molybdenum blue reaction and the induced basiphilia in muscle to a level similar to that obtained in collagen. This appears to confirm the view of Puchtler and Isler that the basiphilia after treatment with PMA is due to free acid groups of the complex acid itself and not to tissue acid groups released by binding of the complex acid to basic groups. TABLE I Molybdenum blue after PMA Basic dye after PMA or PTA Methylation before PMA treatment and molybdenum blue reaction Deamination before PMA treatment and molybdenum blue reaction Acetylation before PMA treatment and molybdenum blue reaction Collagen Muscle Nuclei Red bloodcorpuscles +++ + (+) 0 +++ + (+) O +++ +++ +++ + -T ++ or + + 0 + 0 0 + 0 or O + + + denotes a very strong reaction, + + and + reactions of less intensity. ( + ) indicates a very faint reaction, probably inconsistent from one part of the section to another, and O the complete absence of any reaction. Because of the employment of mercuric chloride as mordant in the PTAH technique and of chromium trioxide in the method of Hrsel (1957), the effects of these two reagents were investigated further. It has been pointed out that mercuric chloride increases the staining intensity of both muscle and collagen with PTAH, particularly intensifying the blue colour in muscle. In addition, if a section which has been methylated or subjected to alkaline hydrolysis or performic acid treatment is exposed to mercuric chloride before staining with PTAH, the normal blue staining of muscle is restored. If, on the other hand, the pretreatment is carried out after the mercurial mordanting, the muscle stains red. Treatment of a section with mercuric chloride before submitting it to PMA increases the intensity of the resulting basiphilia and molybdenum blue reaction in collagen, but greatly reduces their intensity in muscle. Similar effects are obtained when chromium trioxide is used in place of mercuric chloride, and this appears to be the basis of the mordanting in Hrsel's method. If the chromium trioxide is omitted methylated musclefibres stain with the light green of Hrsel's method, but if the methylation is followed by treatment with chromium trioxide the normal staining with eosin is restored. A similar result occurs after performic acid treatment, which, 318 Buhner—Phosphotungstic acid j haematoxylin without chromium mordanting, converts the red staining of muscle to green. The effect of performic acid on staining with Hrsel's method is, like its effect on PTAH staining, reproduced by treatment with formic acid alone, and this casts some doubt on Hrsel's conclusion that the effect of performic acid treatment is due to a specific destruction of tryptophane. Moreover, in a section which has been exposed to performic acid for twice the period necessary for complete abolition of the DMAB-nitrite reaction (Adams, 1957), the eosin staining of muscle with Hrsel's method is restored by mordanting with chromium trioxide or mercuric chloride. In an attempt to shed further light on the staining mechanism with PTAH, a study was made of the staining mixture itself. PTAH is deep purple and has a pH of about 2-5. Addition of strong acid turns it bright red; addition of alkali first turns it blue, but with further alkali the dye lake may be broken. Addition of glycine to PTAH produces little change in colour; tryptophane, and to a less marked extent serine, change the colour towards blue, while lysine and histidine induce the formation of a deep blue precipitate. The reactions of the latter two amino-acids are presumably associated with their basicity, while the reaction with tryptophane may be due to reduction of the PTA, in a comparable manner to the reduction of Folin's phenol reagent by tryptophane (Herriott, 1947). If the PTAH mixture is boiled it becomes light red, but the original purple colour is restored on cooling. Paper chromatography, with water or dilute acetic acid as dispersion medium, distinguishes a more rapidly diffusing blue colour from a more slowly diffusing red. This is comparable with chromatography of Mallory's PTA / aniline blue / orange G mixture, where the rapidly diffusing orange is separated from the more slowly diffusing blue. Electrophoresis of PTAH through an agar gel, as described by Baker (1958), shows that both its red and blue components are anionic. Sections stained with PTAH are extremely fast to treatment with strong acid. Dilute alkali rapidly elutes the stain, more rapidly with the red than the blue, while a 6 M solution of urea removes the red but leaves the blue intact. This may be compared with the behaviour of sections stained with aniline blue. Used as a 1% solution in acetic acid, aniline blue stains all tissues but has a particularly strong affinity for collagen. The stain is eluted by 6 M urea much more rapidly than by alkaline solutions of similar pH. In conjunction with either PMA or PTA, staining with aniline blue is slower and less intense, but the dye is now bound specifically to such structures as collagen. Here also it is rapidly eluted by urea solution, though the complex acid remains bound to the tissues and can be demonstrated by the molybdenum blue reaction or by the increase of basiphilia. Differential staining with PTAH is dependent upon the relative proportions of PTA and haematein in the staining mixture. If a mixture is made containing ten times the usual proportion of haematoxylin to PTA, comparable in constitution to the phosphomolybdic acid-haematoxylin mixture of Mallory, it is Buhner—Phosphotungstic acid / haematoxylin 319 of a deep, bluish purple colour which, like PMAH, stains all tissue components the same colour. On the other hand, a great increase in the proportion of PTA results in a light red mixture, which colours tissue sections only very faintly. Discussion The results indicate that the different staining methods which have been investigated are fundamentally similar. Tissues such as red cells, which do not bind the complex acids in a demonstrable form in formalin-fixed paraffin sections, do not stain with PTAH and stain only with the eosin of Hrsel's technique and the orange G of Mallory's PTA / aniline blue / orange G mixture. Collagen, which binds the complex acids strongly, stains red with PTAH and stains with the light green of HrSel's technique and the aniline blue of Mallory's mixture. Muscle-fibres and nucleoli bind the complex acids less strongly than collagen, stain blue with PTAH and take the eosin of Hrsel's technique and the orange G of Mallory's mixture. With all the methods, however, muscle tends to take an element of the fibre stain unless some pretreatment, such as mercurial or chromium mordanting, is employed. Methylation or treatment with dilute alkali makes the staining of muscle resemble the normal staining of collagen unless mercurial or chromium mordanting is interposed. Alkaline hydrolysis makes the staining of red cells resemble that of untreated muscle-fibres, while prolonged methylation makes them stain like collagen fibres. The hypotheses suggested by other workers will be considered first. There is no evidence to support the view of Monne and Slautterback (1951) that the fibre stain of trichrome methods binds to the amino-groups of amino-sugars and the plasma stain to protein-bound amino-groups, other than the fact that certain mucoprotein materials do take the fibre stain. Although it is impossible to refute this hypothesis completely, because of the lack of specific histochemical reactions for amino-sugars, the effects of mordanting and blocking techniques are difficult to reconcile with it. The opinion of Landing and his colleagues (1952), that PMA reacts specifically with choline, does not appear to be justifiable, as there are many other tissue constituents with which the complex acids might theoretically be expected to react. Lipid extraction does not affect subsequent staining with PTAH. The results reported by Puchtler and Isler (1958) on the tissue binding of PMA have been largely confirmed by the present investigation, though, as will be pointed out later, their explanation of the staining of collagen with aniline blue in the presence of complex acid is not the only one to fit the available facts. The view of Hrsel, who considered that eosin staining with his technique is associated with the presence of tryptophane, merits some consideration. It might similarly be suggested that blue staining with PTAH indicates the presence of protein-bound tryptophane, though proteins that contain tryptophane may require pretreatment with alkali, as with red cells, or potassium 320 Buhner—Phosphotungstic acid j haematoxylin dichromate, as with fibrin (Pearse, 1960), before staining blue. Such an explanation did indeed present itself early in this investigation, when the effect of pretreatment with performic acid was first noticed. The similar effect of formic acid alone, however, together with the restoration of the blue staining of muscle by mercurial or chromium mordanting, indicates that the alteration of staining after performic acid is not due to a specific destruction of tryptophane. The production of a blue colour when tryptophane is added to the PTAH mixture and the known reduction of Folin's reagent by tryptophane suggest that there may be a characteristic reaction with PTAH, but this is insufficient basis for a hypothesis. Moreover, Hrsel himself pointed out exceptions to his rule, mentioning proteins that contain tryptophane but stain with the light green of his technique. The most satisfactory explanation of the differential staining with PTAH and of its alteration by the various pretreatments which have been employed in this investigation appears to be that suggested by Baker (1958) for staining with acid dyes generally. Thus, the red PTAH complex seems to be of larger molecular size than the blue complex and, correspondingly, the aniline blue of Mallory's mixture is of larger molecular size than the orange G. Collagen is permeated by the largest molecules, the red PTAH and the aniline blue, and muscle by the smaller blue PTAH complex and the orange G. Red cells are permeated ohly by the orange G, which is presumably of still smaller molecular size than the blue PTAH complex. The effects of some of the pretreatments which were employed may be due simply to interference with the configuration of the protein molecule rather than to removal of any specific reacting group. Alkaline hydrolysis, for instance, appears to 'open up' the muscle protein sufficiently for the entry of the largest molecules, and the red cell protein sufficiently for the entry of the intermediate blue PTAH complex. The effect of methylation may possibly have a similar basis. The actions of chromium mordanting in preventing the element of fibre stain in muscle and in restoring normal staining of muscle after methylation or alkaline hydrolysis may be due to the formation of cross-linkages in the tissue protein, reducing its permeability. On the other hand, mercurial mordanting has the same effects on the staining picture, though mercury is said not to form crosslinkages (Pearse, i960). However, the reactions of mercuric chloride with tissue proteins are far from clear (Baker, 1958). It might be expected that if the configuration of a protein permits the entry of a particular dye or dye complex, certain specific groups which will bind the dye must also be present for staining to occur. The results of the blocking techniques give no very firm evidence of what groups are involved in the binding of the different dyes. The failure of nitrosation to have any significant effect on the staining of the thicker collagen fibres suggests the participation there of groups other than primary and secondary amines. The very gross impairment in the staining of collagen produced by benzoylation indicates that other basic groups and hydroxyl groups may be involved, though, as Lillie (1958) points out, the effects of benzoylation seem to be more far- Buhner—Phosphotungstic acid j haematoxylin 321 reaching than a simple blockade of basic and hydroxyl groups. There does, however, seem to be some variation between different tissues in their responses to blocking techniques. Thus, the staining of fine collagen fibres is considerably reduced by nitrosation, while Monne and Slautterback (1951) found that deamination completely abolished aniline-blue staining in the yolk of sea-urchin eggs. It is possible that amino-groups and other groups are capable of binding the red PTAH complex or the aniline blue of Mallory's mixture, but that in fine collagen fibres and in the yolk of sea-urchin eggs the amino-groups predominate while in thicker collagen fibres other groups are more important. Alternatively, and much more probably, the basic groups of the thick collagen fibres may simply be more resistant to nitrosation, either for physical or chemical reasons. It has been pointed out by Terner and Clark (i960) that substitution in amino-groups may make them resistant to nitrosation, though they continue to bind acid dyes. The hydroxyl groups of hydroxy-proline may possibly be involved in the binding of the red PTAH complex to collagen, but the elution of the PTAH by strong urea solution is hardly sufficient evidence to suggest that hydrogen bonding is concerned in this when mild alkaline hydrolysis is equally effective. However, the rapid elution of aniline blue by urea solution suggests that hydrogen bonding may be involved in the binding of aniline blue to collagen. Gustavson (1957) has considered that the amphionic dye benzopurpurine 4B is held by hydrogen bonding to the hydroxyl groups of collagen, and a similar explanation may account for the great affinity of aniline blue for collagen. Puchtler and Isler (1958) explained the binding of aniline blue to collagen in the presence of PMA by suggesting that the dye was held by free acidic groups of tissue-bound complex acid. There is no direct evidence that this is true, or that the reduction in the staining of collagen by aniline blue when the dye is used in conjunction with complex acid instead of in simple acid solution is not due to competition for binding sites between dye and polyacid (Baker, 1958). The failure of muscle to stain with short exposure to PTAH after acetylation, phosphorylation and deamination implies that amino-groups and probably other basic groups and hydroxyl groups are involved in the binding of the blue PTAH complex. The conversion of the blue staining to red by methylation or alkaline hydrolysis may be due, as has already been suggested, to rupture of intramolecular bonds in the muscle protein. Obviously methylation, which has been claimed to be specific for carboxyl groups (FraenkelConrat and Olcott, 1945), would be expected to affect linkages between carboxyl groups and other groups. The very mild and brief alkaline hydrolysis may break ionic linkages, and the similar though less complete effect of urea solution suggests that rupture of hydrogen bonds may be involved in the alteration of the staining reaction of muscle. On the other hand, it is possible that carboxyl groups are necessary for the binding of the blue complex to muscle, and this is suggested by the partial restoration of blue staini ng after permanganate 'demethylation'. One would have to suppose, then, t hat the 322 Buhner—Phosphotungstic acid / haematoxylin carboxyl groups of red cells are less easily methylated than those of muscle. Moreover, if carboxyl groups are essential for the binding of the blue PTAH complex, it is difficult to explain the increased blue staining after mercurial or chromium mordanting—which, at any rate according to some authorities (Pearse, i960)—would be expected to block carboxyl groups. There is, however, an indication that acidic groups may be involved in the binding of complex acid to nuclei, in that treatment with PMA or PTA greatly reduces the normal nuclear basiphilia. After methylation, treatment with complex acid induces a nuclear basiphilia comparable with that occurring in collagen. The blue and the red staining with PTAH must be due to different polyacid / dye complexes and in some respects the PTAH mixture resembles the lakes of PTA with basic dyes (Pratt, 1947). Thus an increase in polyacid content or heating of the mixture lightens the colour. In addition, the similarity of the staining distribution of the red PTAH complex with that of the basic thiazine dyes after pretreatment of the section with PMA or PTA suggests that the red PTAH may be a complex of similar structure to the lakes of PTA with basic dyes. PTA might be expected to bind to haematein in this way, since haematein will be positively charged when the pH is below its isoelectric point of about 6-5 (Baker, 1958). Possibly the blue complex is of a similar nature, but with a lower polyacid content and molecular size. On the other hand, PTA may form complexes of another type with haematein, similar to the complexes of tungstic acid with polyhydroxy-phenols which, because of their stability, are presumed to contain chelate linkages (Sidgwick, 1950). The blue PTAH complex may be of this nature, though it is of course likely that both red and blue staining are each due to mixtures of several different complexes. No complete explanation can be offered for the staining reactions of tissue tonstituents with the various preparations which have been studied, but it is clear that differential staining with all of them is dependent upon similar fundamental principles. The most satisfactory approach appears to be that of relating the staining by different dye complexes with the permeability of tissue proteins to molecules of differing sizes, as Baker has described. Tissue groups must also be available to bind the dye complex once it has permeated the protein, but until more specific chemical techniques can be applied it is impossible to define these precisely. In conclusion it is worth emphasizing that the results of blocking techniques in an investigation of this kind must be interpreted with caution. Effects on staining reactions may often be due to interference with the normal configuration of the protein molecule rather than to blockade of a specific reactive group. My thanks are due to Professor R. D. Lockhart, in whose Department the initial part of the work was carried out, and to Professor G. A. G. Mitchell for his valuable advice on the preparation of this paper. Buhner—Phosphotungstic acid / haematoxylin 323 References ADAMS, C. W. M., 1957. J. clin. Path., 10, 56. BAKER, J. R., 1958. Principles of biological microtechnique. London (Methuen). FRAENKEL-CONRAT, H., and OLCOTT, H. S., 1945. J. biol. Chem., 161, 259. GREEN, R. W., ANG, K. P., and LAM, L. C , 1953. Biochem. J., 54, 181. GUSTAVSON, K. H., 1957. In Connective tissue; a symposium, edited by R. E. Tunbridge. Oxford (Blackwell). HERRIOTT, R. M., 1947. Advances in Protein Chemistry, 3, 169. HR§EL, I., 1957. Acta histochem., 4, 47. JONES, B. M., i960. Proc. R. phys. Soc. Edin., 28, 35. LANDING, B. H., UZMAN, L. L., and WHIPPLE, A. (1952). Lab. Invest. 1, 456. LILLIE.R. D., 1954. Histopathologic technic andpractical histochetnistry. New York (Blakiston). 1958. J. Histochem. Cytochem., 6, 352. LISON, L., 1955. Quart. J. micr. Sci., 96, 327. MALLORY, F. B., and PARKER, F., 1929. In Handbook of microscopical technique, edited by C. E. McClung. New York (Hoeber). MONNE, L., and SLAUTTERBACK, D. B., 1951. Ark. Zool., 1, 455. PEARSE, A. G. E., i960. Histochemistry, theoretical and applied, London (Churchill). PEERS, J. H., 1941. Arch. Path. Lab. Med., 32, 446. PRATT, L. S., 1947. TJie chemistry and physics of organic pigments. New York (Wiley). PUCHTLEB, H., and ISLER, H., 1958. J. Histochem. Cytochem. 6, 265. SIDGWICK, N. V., 1950. The chemical elements and their compounds. Oxford (Clarendon Press). SPEAKMAN, J. B., 1955. In Fibrous proteins and their biological significance. Symposium of Society for Exp. Biol., IX, edited by R. Brown and J. F. Danielli. Cambridge (University Press). TERNER, J. Y., and CLARK, G., i960. J. Histochem. Cytochem., 8, 184.