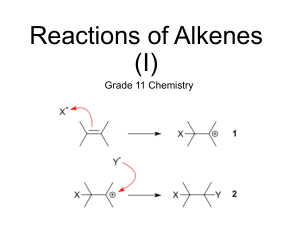
Согласно методу молекулярных орбиталей электронная конфигурация невозбуждённой молекулы CO σ2Oσ2zπ4x, y σ2C. Тройная связь образована σ-связью, образованной за счёт σz электронной пары, а электроны дважды вырожденного уровня πx, y соответствуют двум πсвязям. Электроны на несвязывающих σC-орбитали и σO-орбитали соответствуют двум электронным парам, одна из которых локализована у атома углерода, другая — у атома кислорода. Молекула слабо поляризована, её электрический дипольный момент μ = 0,04⋅10−29 Кл·м. Многочисленные исследования показали, что отрицательный заряд в молекуле CO сосредоточен на атоме углерода C−←O+ (направление дипольного момента в молекуле противоположно предполагавшемуся ранее). Цианиды - непрочные соединения, при длительном воздействии содержащегося в воздухе СO2 цианиды разлагаются 2KCN+C02+H20=K2C03+2HCN. (CN)2 - дициан (N=C–C=N) – бесцветный ядовитый газ; с водой взаимодействует с образованием циановой (HOCN) и синильной (HCN) кислот: (HCN) кислот: (CN)2 + H2O HOCN + HCN. В этой, как и в реакции, приведенной ниже, (CN)2 похож на галоген: СО + (CN)2 CO(CN)2 (аналог фосгена). Циановая кислота известна в двух таутомерных формах: H–N=C=O H–O–C=N Изомером является кислота H–O=N=C (гремучая кислота). Соли HONC взрывают (используются как детонаторы). Родановодородная кислота HSCN - бесцветная, маслянистая, летучая, легко затвердевающая (Тпл=278 К) жидкость. В чистом состоянии очень неустойчива, при ее разложении выделяется HCN. В отличие от синильной кислоты HSCN достаточно сильная кислота (К=0,14). Для HSCN характерно таутомерное равновесие: H–N=С=S H–S–C=N SCN– ион кроваво-красного цвета (реактив на ион Fe3+). Производные от HSCN соли-роданиды - легко получить из цианидов путем присоединения серы: KCN+S=KSCN Большинство роданидов растворимо в воде. Нерастворимы в воде соли Hg, Au, Ag, Си. Ион SCN-, как и CN-, склонен давать комплексы типа Мз1 M" (SCN)6, где M''Cu, Mg и некоторые другие. Диродан (SCN)2 - светло-желтые кристаллы, плавящиеся 271 К. Получают (SCN)2 по реакции 2AgSCN + Br2 2AgBr+ (SCN)2. Из других азотсодержащих соединений следует указать цианамид и его производное - цианамид кальция CaCN2 (Ca=N–C=N), который используется в качестве удобрения There are a myriad of organic compounds containing carbon-nitrogen bonds, including: amines, imines, and nitriles. However, here we are concerned with the simplest carbon-nitrogen compounds. Cyanogen Cyanogen, (CN)2, may be considered the smallest molecular fragment containing carbon and nitrogen (Figurea). The reaction chemistry of cyanogen is related to that of the halogens, i.e., F2, Cl2, etc. Consequently, cyanogen is called a pseudo halogen. The structures of (a) cyanogen, (CN)2 and (b) dicyanoacetylene (carbon subnitride, C4N2). As shown in Table the bonding in cyanogen is consistent with localization of the π-bonding between carbon and nitrogen given the similarity of the C-N bond distance in cyanogens and acetonitrile. However, there is clearly some π-delocalization associated with the C-C distance given its shortening as compared to ethane. A comparison of the bond distances in selected carbon nitrogen compounds. Compound Formula C-C bond distance (Å) C-N bond distance (Å) Cyanogen (CN)2 1.393 1.163 Hydrogen cyanide HCN 1.154 Acetonitrile CH3CN 1.46 1.16 Ethane C 2 H6 1.535 Ethylene C 2 H4 1.339 Cyanogen is produced by the reaction of a mixture of the cyanide and chloride of mercury, Equation. Alternatively, the decomposition of unstable copper(II) cyanide, formed from a copper(II) salts with a Group 1 cyanide, Equation, yields cyanogens, Equation. Cyanogen is a flammable gas (Mp = -28 °C and Bp = -21 °C) that produces the second hottest flame natural flame (after carbon subnitride, C4N2) with a temperature of over 4525 °C when burnt in oxygen. Heating cyanogen in the absence of oxygen results self polymerizes, Equation. Hydrolysis of cyanogen results in addition across the carbon-nitrogen triple bonds and the formation of oxamide, Equation. Cleavage of the C-C bond does occur in the presence of base (e.g., KOH), with the formation of cyanide (CN-) and cyanate (CNO-) salts, Equation. Dicyanoacetylene Dicyanoacetylene (also known as carbon subnitride or by its IUPAC name but-2-ynedinitrile) has the structure shown in Figureb, and may be thought of as a dicyanaide substituted acetylene. At room temperature, dicyanoacetylene is a clear liquid, however, solid dicyanoacetylene has been detected in the atmosphere of Titan (the largest moon of the planet Saturn) by infrared spectroscopy. Dicyanoacetylene is an entropic explosive giving carbon powder and nitrogen gas. In the presence of oxgyen it burns with a bright blue-white flame at a temperature of 4990 °C. Hydrogen cyanide Hydrogen cyanide (HCN) is a colorless, highly poisonous, gas (Mp = -13.5 °C and Bp = 25.6 °C). Due to its original isolation from Prussian blue (hydrated ferric ferrocyanide), hydrogen cyanaide is also known by the name of prussic acid. The synthesis of hydrogen cyanide is accomplished commercially by the partial oxidation of methane in the presence of ammonia, Equation, using a platinum catalyst. The heat to activate the reaction is derived from the partial combustion of the methane and ammonia. The resulting aqueous solution is dried by distilled from phosphorus pentoxide (P2O5) to yield anhydrous hydrogen cyanide. Hydrogen cyanide may also be formed in the absence of oxygen, Equation; however, in this case the reaction must be heated externally. Small quantities of hydrogen cyanide for laboratory use may be prepared by the reaction of an acid with a cyanide salt (either potassium or sodium), Equation. The structure of hydrogen cyanide is shown in Figure along with its isomeric form, hydrogen isocyanide (HNC). While hydrogen cyanide is present in the pits of many fruits, and is generated by burnet moths and some millipedes, hydrogen isocyanide is only found in interstellar space. It is postulated, however, that along with HCN, HNC is an important building block for amino acids and hence life. The structures of (a) hydrogen cyanaide (HCN) and (b) its isomer hydrogen isocyanide (HNC). In the liquid state hydrogen cyanide forms strong hydrogen bonds (Figure). Hydrogen cyanide is a good solvent for polar compounds due to its high permittivity (∈r) and high dipole moment (2.98 D). The hydrogen bonding in liquid hydrogen cyanide. In aqueous solution hydrogen cyanide is a weak acid, Equation, and several salts are known. However, HCN also reacts with water to give ammonium formate via formamide, Equation. In a similar manner to cyanogen’s relationship to the halogens, the cyanide anion (CN-) is considered a pseudo halide (i.e., F-, Cl-, etc), and as such forms many coordination compounds, e.g., [Fe(CN)6]3- and [Ag(CN)2]-. Bibliography J. Wu and N. J. Evans, Astrophys. J., 2003, 592, L79. Carbamic acid (other names aminomethanoic acid) is the compound with the formula H2NCOOH. The attachment of the acid group to a nitrogen or amine (instead of carbon) distinguishes it from carboxylic acid and an amide. Many derivatives and analogues of carbamic acid are known. They are generally unstable, reverting to the parent amine and carbon dioxide.[1] The deprotonated anion (or conjugate base) of this functional group is a carbamate. Carbamic acid is a planar molecule.[2] Carbamic acid is an intermediate in the production of urea, which involves the reaction of carbon dioxide and ammonia.[3] CO2 + NH3 → H2NCOOH H2NCOOH + NH3 → CO(NH2)2 + H2O Carbamoyltransferases are transferase enzymes classified under EC number 2.1.3. Derivatives of carbamic acid Carbamic acids are intermediates in the decomposition of carbamate protecting groups; the hydrolysis of an ester bond produces carbamic acid the evolution of carbon dioxide drives the deprotection reaction forward, yielding the unprotected amine. Carbamates usually refer to esters of carbamic acid. Methyl carbamate is the simplest ester of carbamic acid. Unlike carbamic acids, the esters are stable. They are prepared by reaction of carbamoyl chlorides with alcohols, the addition of alcohols to isocyanates, and the reaction of carbonate esters with ammonia.[4] Some esters have use as muscle relaxants which bind to the barbiturate site of the GABAA receptor,[5] while others are used as insecticides, for example aldicarb.[6] Carbamoyl phosphate is an anion of biochemical significance. In land-dwelling animals, it is an intermediary metabolite in nitrogen disposal through the urea cycle and the synthesis of pyrimidines. Its enzymatic counterpart, carbamoyl phosphate synthetase I (CPS I), interacts with a class of molecules called sirtuins, NAD dependent protein deacetylases, and ATP to form carbamoyl phosphate. CP then enters the urea cycle in which it reacts with ornithine (a process catalyzed by the enzyme ornithine transcarbamylase) to form citrulline. A defect in the CPS I enzyme, and a subsequent deficiency in the production of carbamoyl phosphate has been linked to hyper-ammonemia in humans.[1] Production It is produced from bicarbonate, ammonia (derived from amino acids), and phosphate (from ATP). The synthesis is catalyzed by the enzyme carbamoyl phosphate synthetase, as follows: HCO−3 + ATP → ADP + HO–C(O)–OPO2−3 (carboxyl phosphate) HO–C(O)–OPO2−3 + NH3 + OH− → HPO2−4 + −O–C(O)NH2 + H2O − O–C(O)NH2 + ATP → ADP + H2NC(O)OPO2−3 Ammonium carbamate is the inorganic compound with the formula NH4[H2NCO2]. This salt is formed by the reaction of ammonia with carbon dioxide, and is a white solid that is extremely soluble in water, less so in alcohol. It is unusual in that it will degrade at room temperature. Preparation and structure It is prepared by the direct reaction between liquid ammonia and dry ice (solid carbon dioxide):[2] 2 NH3 + CO2 → H2NCOONH4 The structure of solid ammonium carbamate has been confirmed by X-ray crystallography. The oxygen centers form hydrogen bonds to the ammonium cation.[3] Reactions Ammonium carbamate reverts to carbon dioxide and ammonia even as a solid:[4] NH2CO2NH4 → 2NH3 + CO2 It hydrates reversibly:[4] NH2CO2NH4 + H2O → (NH4)2CO3 Ammonium carbamate serves a key role in the formation of carbamoyl phosphate, which is necessary for both the urea cycle and the production of pyrimidines. In this enzyme-catalyzed reaction, ATP and ammonium carbamate are converted to ADP and carbamoyl phosphate:[5][6] ATP + NH2CO2NH4 → ADP + H2NC(O)OPO32− Uses The ability of ammonium carbamate to make urea was first discovered in 1870 when Bassarov heated ammonium carbamate in sealed glass tubes at temperatures ranging from 130 to 140 °C. This heating yields urea and water in an equimolar ratio.[4] A typical industrial plant that makes urea can produce up to 1500 tons a day. Ammonia and carbon dioxide is excessively fed to a synthesis reactor in this process. Ammonium carbamate is produced as an intermediate in this reactor and can then be dehydrated to urea according to the following equation:[7] NH2CO2NH4 → NH2CONH2 + H2O Ammonium carbamate has also been approved by the Environmental Protection Agency as an inert ingredient present in aluminium phosphide pesticide formulations. This pesticide is commonly used for insect and rodent control in areas where agricultural products are stored. The reason for ammonium carbamate as an ingredient is to make the phosphine less flammable by freeing ammonia and carbon dioxide to dilute hydrogen phosphine formed by a hydrolysis reaction.[8] Ammonium carbamate can also be used as a good ammoniating agent, though not nearly as strong as ammonia itself.[9] Степень окисления Азота в соединениях Азот образует двухатомные молекулы состава N2 за счет наведения ковалентных неполярных связей, а, как известно, в соединениях с неполярными связями степень окисления элементов равна нулю. Для азота характерен целый спектр степеней окисления, среди которых есть как положительные, так и отрицательные. Степень окисления (-3) азот проявляет в соединениях под названием нитриды (Mg+23N-32, B+3N-3), самым известным из которых является аммиак (N-3H+13). Степень окисления (-2) азот проявляет в соединениях перикисного типа – пернитридах, простейшим представителем которых является гидразин (диамид/ пернитрид водорода) – N-22H2. В соединении под названием гидроксиламин – N-1H2OH–азот проявляет степень окисления (-1). Наиболее устойчивые положительные степени окисления азота – это (+3) и (+5). Первую из них он проявляет во фториде (N+3F-13), оксиде (N+32O-23), оксогалогенидах (N+3OCl, N+3OBr и т.д.), а также производных аниона NO2— (KN+3O2, NaN+3O2 и др.). Степень окисления (+5) азот проявляет в оксиде N+52O5, оксонитриде N+5ON, диоксофториде N+5O2F, а также в триоксонитрат (V) –ионе NO3— и динитридонитрат (V) (азид) –ионе NN2—. Азот также проявляет степени окисления (+1) – N+12O, (+2) – N+2O и (+4)N+4O2 в своих соединениях, но значительно реже.