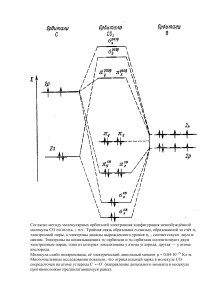
MSC 93A30 DOI: 10.14529/mmp180206 MATHEMATICAL MODEL OF GAS HYDRATE OF HYDROGEN SULFIDE FORMATION DURING ITS INJECTION INTO A NATURAL LAYER M.Ê. Khasanov1 , G.R. Rakova2 Sterlitamak branch of Bashkir State University, Sterlitamak, Russian Federation, Mavlyutov Institute of Mechanics UFRC RAS, Ufa, Russian Federation E-mail: hasanovmk@mail.ru, rakova_guzal@mail.ru 1 2 The mathematical model of liquid hydrogen sulde injection into the semi-innite porous layer saturated with the oil and water accompanied by H2 S gas hydrate formation is presented here. We considered the case when the hydrate formation occurs at the frontal border and the oil displacement's front by hydrogen sulde is ahead of this boundary. Solutions for pressure and temperature in every layer's area are built by help of the selfsimilar variable formation method. The values of the parameters of the moving interphase boundaries are found as the result of the iteration procedure. The coordinate dependence of phase boundaries on the injection pressure was studied on the basis of the obtained solutions. We have established that for the existence of solution with two dierent interphase boundaries, the injection pressure must be above a certain limiting value. The dependence of the limiting value of pressure on the initial temperature of the layer at dierent temperatures of the injected hydrogen sulphide is constructed. The results of the calculations showed that the constructed mathematical model with three areas in the reservoir gives an adequate description of the process at high injection pressures, the temperature of the injected hydrogen sulde and the initial temperature of the layer. Keywords: mathematical model; self-similar variable solution; porous medium; ltration; gas hydrates; hydrogen sulde. Introduction One of the methods for the emission's reducing of hydrogen sulde generated by industrial facilities into the atmosphere is its underground disposal in the exhausted hydrocarbon deposits [1, 2]. As there is a risk of the leaking of the recyclable gases in the form of a uid to the surface at their long-term underground storage we consider the possibility of their transformation into the gas hydrate state which allows the storing of the same gas number at much lower pressures compared to its free state [3, 4]. As any technological ideas need to be backed up by the relevant calculations based on the theoretical models, the construction of the adequate mathematical models of hydrate formation in the natural layers is an actual task. The mathematical models of hydrate formation in the porous layers saturated with methane and water are represented in the works [5-8]. The mathematical model of H2 S hydrate formation in the layers saturated with oil and water during injection of uid hydrogen sulde are represented in the work [9]. But in the above mentioned works the task is achieved due to its simple determination when the gas hydrate formation occurs at the border coinciding with the oil replacement's border by hydrogen sulde. The mathematical model of H2 S gas hydrate formation is represented in this work the hydrate formation at the front border that doesn't coincide with the oil replacement's border by the uid hydrogen sulde. Âåñòíèê ÞÓðÃÓ. Ñåðèÿ ≪Ìàòåìàòè÷åñêîå ìîäåëèðîâàíèå è ïðîãðàììèðîâàíèå≫ (Âåñòíèê ÞÓðÃÓ ÌÌÏ). 2018. Ò. 11, 2. Ñ. 7382 73 M.K. Khasanov, G.R. Rakova 1. Problem Statement The conditions of gas hydrate of hydrogen sulde existence are represented in the phase diagram (Fig. 1) [6]. The curve line gh determines in this diagram the three-phase balancing of water, gas hydrogen sulde and its gas hydrate, the curve line lh shows the balancing of water, uid hydrogen sulde and its gas hydrate, the curve line lg represents two-phase balancing of uid and gas hydrogen sulde. Suitably the gas hydrate of hydrogen sulde is to the left of the curve lines gh and lh. All four indicated phases are balanced in the quadrupole point Q (TQ =302,6 Ê and pQ =2,24 ÌPà). p, ÌPà lh 10 5 Q lg 1 0.5 0.1 273 gh T, Ê 283 293 303 Fig. 1. Phase diagram of H2 S-H2 0 system Imagine the semi-nite horizontal porous layer (occupied semi-areas x>0) is saturated with water source saturation Sw0 and oil in the initial moment. We assume that the initial temperature of the layer T0 is higher than the temperature TQ of the accordance quadrupole point. Therefore the problem in question the initial position of the layer doesn't meet the formation conditions of the gas hydrate of hydrogen sulde. Suppose that the uid hydrogen sulde is pumped through the border (x=0) and the pe pressure as well as the Te temperature are in accordance with the conditions of the gas hydrate of hydrogen sulde. The initial and border conditions are Sw = Sw0 , T = T0 , p = p0 (x ≥ 0) when t = 0, and the border conditions are T = Te , p = pe (t > 0) when x = 0. We consider the model with the oil piston displacement by hydrogen sulde as well as the case when the value of the initial water saturation of the layer isn't higher than 0,2 (i.e. the water is immobile) in the work. Also we consider the time scales that are much higher than the characteristic time of the process of hydrate formation kinetics. So we can suppose that hydrate formation occurs at the front border which doesn't coincide with the oil displacement border by the hydrogen sulde. Therefore three characteristic areas appear in the layer in accordance with this case. The pores are saturated with hydrogen sulde and its hydrate in the rst (nearest) area, the water and the hydrogen sulde are in the second (intermediate) area, the pores saturated with oil and water are in the third (remote) one. Accordingly, there are two movable interfacial surfaces: between the rst and the second areas where the water transforms into the gas hydrate state completely (front hydrate formation) and between the second and the third areas where the oil displacement by hydrogen sulde occurs (displacement front). 74 Bulletin of the South Ural State University. Ser. Mathematical Modelling, Programming & Computer Software (Bulletin SUSU MMCS), 2018, vol. 11, no. 2, pp. 7382 ÌÀÒÅÌÀÒÈ×ÅÑÊÎÅ ÌÎÄÅËÈÐÎÂÀÍÈÅ 2. Basic Equations Let's assume the following simplifying assumptions: the porosity of the layer m is constant, the body of the porous medium, gas hydrate as well as the water are incompressible and immobile. H2 S gas hydrate is a double-component system with G mass concentration of the hydrogen sulde. We assume that the uid hydrogen sulde and the oil are weakly compressible uids. Basic equation system describing in the one-dimensional case the ltration and heat transfer process in the porous medium represents the laws of mass energy conservation, Darcy law and equation of state [10]: ∂ ∂ (ρi mSi ) + ∂x (ρi mSi υi ) = 0, ∂t ρc ∂T + ρ c mS υ ∂T = ∂ (λ ∂T ) , i i i i ∂x ∂t ∂x ∂x (1) ∂p k i mS υ = − , i i µi ∂x ρ = ρ (1 + β (p − p )) . i 0i i 0 Where t is time; x is coordinate; m is porosity; p is pressure; T -is temperature; low indexes i=s,l refer respectively to the parameters of hydrogen sulde and oil; ρi is density; ki is phase permeability; υi is actual average speed; ci is specic heat capacity; µi is dynamic viscosity; βi is compressibility factor; ρc è λ are eective values of the volumetric heat capacity and thermal conductivity of the layer saturated. Since the main contribution to the value ρc and λ includes the corresponding parameters of the rock we assume them as the constant values. The phase coecient dependence of the ki permeability on the S(i) saturation and absolute permeability k0 we dene as follows: ki = k0 Si (i = s, l). The total transition of the water into the hydrate state takes place on the surface x = x(n) dividing the rst and the second areas. Therefore, according to the conditions of the mass heat balance at this boundary we have: ) ( ks(1) ∂p(1) ks(2) ∂p(2) ρh G ρh (1 − G) • − (2) + = mSh + − 1 x (n) , µs ∂x µs ∂x ρ0s ρw • • mSh ρh (1 − G)x (n) = mSw0 ρw x (n) , (3) ∂T(1) ∂T(2) • −λ = mSh ρh Lh x (n) , (4) ∂x ∂x where the value x(n) is the front motion speed of the gas hydrate formation H2 S, G is mass concentration of the hydrogen sulde in the hydrate, Lh is specic heat of H2 S gas hydrate formation, Sw0 is initial water saturation, ρw is water density. Low index n refers to the parameters at the border, dividing the rst and the second areas, the low indexes 1 and 2 refer to the parameters of the rst and the second areas accordingly. We assume the temperature at this border as continuous and equal to the temperature of the quadrupole point TQ . The oil displacement by the hydrogen sulde takes place on the surface x = x(d) dividing the second and the third areas. Therefore we have the subject to the conditions of the oil and the hydrogen sulde mass balance as well as the heat's one at this border: λ − ks(2) ∂p(2) • = m (1 − Sw0 ) x (d) , µs ∂x Âåñòíèê ÞÓðÃÓ. Ñåðèÿ ≪Ìàòåìàòè÷åñêîå ìîäåëèðîâàíèå è ïðîãðàììèðîâàíèå≫ (Âåñòíèê ÞÓðÃÓ ÌÌÏ). 2018. Ò. 11, 2. Ñ. 7382 (5) 75 M.K. Khasanov, G.R. Rakova − kl ∂p(3) • = m (1 − Sw0 ) x (d) , µl ∂x (6) ∂T(2) ∂T(3) −λ = 0, ∂x ∂x (7) λ • where the value x(d) is the front motion speed of the oil by the hydrogen sulde. Low index d refers to the parameters at the border, dividing the second and the third areas and the low index 3 refers to the parameters of the third area. The pressure and temperature will be considered continuous variables at both borders. On the basis of the system's equations (1), the equations for the piezoconductivity and the temperature conductivity will be written in the form: ( ) ∂p(i) ∂p(i) (p) ∂ , (8) = χ(i) ∂t ∂x ∂x ∂T(i) ∂ = χ(T ) ∂t ∂x (p) where χ(1) = χ(T ) = λ , ρc X(1) ( ) ∂T(i) ∂x ∂p(i) ∂T 1 + χ(T ) X(i) , 2 ∂x ∂x (9) ks(1) ks(2) kl (p) (p) , χ(2) = , χ(3) = , µs m (1 − Sh ) βs µs m (1 − Sw0 ) βs µl m (1 − Sw0 ) βl ρ0s cs ks(1) ρ0s cs ks(2) ρ0l cl kl =2 , X(2) = 2 , X(3) = 2 . λµs βs λµs βs λµl βl 3. Self-Similar Solution /√ We introduce self-similar variable: ξ = x χ(T ) t. For this variable the equations (8), (9) for the piezoconductivity and temperature conductivity will take the form in every area: ( ) dp(i) d dp(i) −ξ = 2η(i) , (10) dξ dξ dξ ( ) dT(i) dp(i) dT(i) d dT(i) −ξ = X(i) +2 , (11) dξ dξ dξ dξ dξ (p) where η(i) = χ(i) /χ(T ) . After integrating the solutions (10), (11) the solutions for the pressure and temperature distribution in every area can be written in the form: ( pe − p(n) p(1) = p(n) + ( T(1) = T(n) + ( ∫ exp 0 Te − T(n) ) ξ∫(n) ∫ ( exp 2 − 4ηξ(1) ( exp ξ ξ(n) ( ) 2 exp − 4ηξ(1) dξ ξ ξ(n) 0 76 ) ξ∫(n) 2 − ξ4 2 − ξ4 , ) dξ (12) ) − X(1) p(1) dξ ) , − X(1) p(1) dξ Bulletin of the South Ural State University. Ser. Mathematical Modelling, Programming & Computer Software (Bulletin SUSU MMCS), 2018, vol. 11, no. 2, pp. 7382 ÌÀÒÅÌÀÒÈ×ÅÑÊÎÅ ÌÎÄÅËÈÐÎÂÀÍÈÅ ( p(n) − p(d) p(2) = p(d) + T(n) − T(d) ξ exp − 4η(2) dξ ) ξ∫(d) ∫ ( ξ(d) exp ξ(n) ( p(d) − p0 p(3) = p0 + ( ∫∞ ( exp T(d) − T0 ) ∫∞ ξ T(3) = T0 + ∫∞ ) ∫∞ ξ exp (13) ) − X(2) p(2) dξ , − X(2) p(2) dξ ( exp ( 2 − 4ηξ(3) 2 − 4ηξ(3) ) dξ ) , dξ ( 2 ) exp − ξ4 − X(3) p(3) dξ ( ξ(d) 2 − ξ4 ) 2 − ξ4 exp ξ(d) , ) ξ2 ξ T(2) = T(d) + ( ) 2 exp − 4ηξ(2) dξ ( ∫ ξ(d) ξ(n) ( ) ξ∫(d) 2 − ξ4 ) (14) . − X(3) p(3) dξ On the basis of the ratios (2), (4) taking into account the decisions (12), (13) we get the equations for coordinate's determining for the hydrate formation's front ξ(n) and parameter values on it: ( 2 ) ( 2 ) ) ( ( ) ξ ξ(n) p(n) − pe exp − 4η(n) p(d) − p(n) exp − 4η(2) (1) − k = KSh ξ(n) , (15) ks(2) s(1) ξ(d) ξ(n) ( ) ( ) ∫ ∫ 2 2 exp − 4ηξ dξ exp − 4ηξ dξ (2) ξ(n) ( (T(n) −Te ) exp ( ξ(n) ∫ 0 exp ξ2 (n) − 4 −X(1) p(n) 2 − ξ4 −X(1) p(1) (1) 0 ) dξ ) ( − (T(d) −T(n) ) exp ξ(d) ∫ ξ(n) ξ2 (n) − 4 −X(2) p(n) ) ( 2 exp − ξ4 −X(2) p(2) dξ ) = (16) mρh Lh Sh ξ(n) , 2ρc T(n) = TQ , (17) ( ) where K = mµs χ(T ) ρρhs0G + ρh (1−G) −1 . ρw Similarly, on the basis of the ratios (5) to (7) taking into account the decisions (13) and (14) we get the equations system to determine the oil displacement front's coordinate by hydrogen sulde ξ(d) and parameter values on it: ( 2 ) ) ( ξ p(n) − p(d) exp − 4η(d) (2) = mµs χ(T ) (1 − Sw0 ) ξ(d) , (18) ks(2) ξ(d) ( ) ∫ 2 exp − 4ηξ(2) dξ = ξ(n) Âåñòíèê ÞÓðÃÓ. Ñåðèÿ ≪Ìàòåìàòè÷åñêîå ìîäåëèðîâàíèå è ïðîãðàììèðîâàíèå≫ (Âåñòíèê ÞÓðÃÓ ÌÌÏ). 2018. Ò. 11, 2. Ñ. 7382 77 M.K. Khasanov, G.R. Rakova ( kl p(d) − p0 ∫∞ ξ(d) ) ( 2 ) ξ exp − 4η(d) (3) ( ) 2 exp − 4ηξ(3) dξ ( 2 ) ( ) ξ(d) T(d) − T(n) exp − 4 − X(2) p(d) ∫ ξ(d) ξ(n) ( ξ2 ) exp − 4 − X(2) p(2) dξ ( − = mµl χ(T ) (1 − Sw0 ) ξ(d) , ) ( T0 − T(d) exp − ∫∞ ξ(d) ( exp 2 − ξ4 2 ξ(d) 4 (19) ) − X(3) p(d) ) − X(3) p(3) dξ = 0. (20) The system of boundary equations (15) to (20) is presented in the work as following. At the beginning the zero approximation of the desired values at the front of hydrate formation is given. Further, solving the equation (18), we nd the p(d) value (as a function ξ(d) ), substituting that into equation (19) we get the transcendental equation for nding ξ(d) . Have solved this equation (by the half division method) we determine the value ξ(d) (and respectively p(d) ), then from (20) we dene T(d) . Further, substituting (17) into the equation (16) we get the transcendental equation for nding ξ(n) . Solving this equation (by the method of half-division), we determine new approximation of value ξ(n) . Then, solving equation (15), we nd a new approximation of value p(n) . As the result of cyclic repetition of the described iterative procedure we obtain a sequence of approximate values which converges to the target values of the boundary parameters. As a result of cyclic repetition of the described iterative procedure, we obtain a sequence of approximate values Y(k) = (ξ(n) , ξ(d) , p(n) , p(d) , T(d) ). This sequence converges within the metric: ( ( (k+1) ) (k+1) (k) (k+1) (k) ρ Y , Y (k) = max ξ(n) − ξ(n) , ξ(d) − ξ(d) , (k+1) (k) (k) (k) ) (k+1) (k+1) p(n) p(n) p(d) T(d) p(d) T(d) − − − , , . p0 p0 p0 p0 T0 T0 To establish the time of the termination of the iterations, the following condition was used: ( ) ρ Y (k+1) , Y (k) < 0, 001. 4. Result of Calculation The Fig. 2 shows the dependence of the coordinate fronts of the hydrate formation H2 S (curve line1) and the oil displacement by the liquid hydrogen sulde (curve line2) on the injection pressure. Hereinafter, unless otherwise specied for the parameters characterizing the system, the following values are adopted: m = 0, 1, Sw0 = 0, 2, p0 = 8 ÌPà, Te =290 Ê, T0 =305 Ê, k0 = 10−11 m2 , G = 0,24, βs = 3· 10−9 Pà−1 , βl = 1·10−9 Pà−1 , λ = 2 w/(ì·Ê), ρc = 2·106 J/(Ê·kg), µs = 2·10−4 Pà·s, µl = 2·10−3 Pà· s, ρh = 1003 kg/m3 , ρw = 1000 kg/m3 , ρ0s =890 kg/m3 ,ρ0l =900 kg/m3 , cs = 1800 J/(Ê·kg), cl = 1900 J/(Ê·kg), Lh = 4,1·105 J/kg. According to the Fig. 2 the speed of the rst front is almost independent on the injection pressure and the speed of the second front increases with its growth. This is due to the fact that the front speed of the oil displacement by hydrogen sulde is limited by 78 Bulletin of the South Ural State University. Ser. Mathematical Modelling, Programming & Computer Software (Bulletin SUSU MMCS), 2018, vol. 11, no. 2, pp. 7382 ÌÀÒÅÌÀÒÈ×ÅÑÊÎÅ ÌÎÄÅËÈÐÎÂÀÍÈÅ 4 x ( n, d ) 2 3 2 1 1 pe , MPà 0 8.2 8.4 8.6 8.8 9.0 Fig. 2. Dependence of the border coordinates of hydrate formation H2 S (curve line 1) and oil displacement by hydrogen sulde (curve line 2) on the injection pressure 8.50 p* , Ì P à 8.45 2 8.40 1 8.35 8.30 303 T0 , Ê 304 305 306 307 Fig. 3. The dependence of the limiting pressure on the initial temperature in the layer at Te =290 Ê (curve line 1) and 285 Ê (curve line 2) the intensity of mass transfer in the layer that is proportional to the generated in the layer pressure drop according to Darcy's law. And the front speed of the hydrate formation is limited primarily by the dissipation of heat released during the phase transition, i.e. the intensity of heat transfer in the layer. It is also seen that for the suciently low values of injection pressure the coordinates of both fronts are aligned, i.e. the front speed of the oil displacement by the liquid hydrogen sulde is reduced to the speed of the hydrate formation front. Because the original hydrogen sulde in the layer was absent, that in this mode, the speed of movement of united fronts will be limited by the supply of hydrogen sulde, i.e. by the injection pressure. Thus, there is some value of pressure (called limiting pressure) above which the mode with two dierent interfacial borders is implemented under consideration in this work. There are given in the Fig. 3 the dependences of the injection pressure corresponding to the alignment of the coordinates of both fronts, on the initial layer temperature at dierent injection temperatures Te =290 Ê (curve line 1) è 285 Ê (curve line 2). According to the Fig. 3 the given pressure value decreases while increasing the initial layer's temperature and temperature of hydrogen sulde injected. In other words, the mode with two dierent interphase bounders is realized at suciently high layer's temperatures and the hydrogen sulde injected. This is due to the fact that the intensity of heat removal at the border Âåñòíèê ÞÓðÃÓ. Ñåðèÿ ≪Ìàòåìàòè÷åñêîå ìîäåëèðîâàíèå è ïðîãðàììèðîâàíèå≫ (Âåñòíèê ÞÓðÃÓ ÌÌÏ). 2018. Ò. 11, 2. Ñ. 7382 79 M.K. Khasanov, G.R. Rakova of hydrate formation decreases with an increase layer's temperature and the injection and, accordingly, the rate of hydrate formation front decreases. Thus, the front of oil displacement by hydrogen sulde outpaces the front of H2 S gas hydrate formation even at low values of injection pressure at high temperatures of the layer and hydrogen sulde injected. Acknowledgements. This work was supported by the Russian Foundation for Basic Research and the Republic of Bashkortostan (project No. 17-48-020123). References 1. Machel H.G. Geological and Hydrogeological Evaluation of the Nisku Q-Pool in Alberta, Canada, for H2 S and/or CO2 Storage. Oil and Gas Science and Technology, 2005, vol. 60, pp. 5165. DOI: 10.2516/ogst:2005005 2. Xu T., Apps J.A., Pruess K., Yamamoto H. Numerical Modeling of Injection and Mineral Trapping of CO2 with H2 S and SO2 in a Sandstone Formation. Chemical Geology, 2007, vol. 24, no. 34, pp. 319346. DOI: 10.1016/j.chemgeo.2007.03.022 3. Byk S.Sh., Makogon Yu.F., Fomina V.I. 1980. Gazovye gidraty [Gas Hydrates]. Moscow, Khimiya, 4. Duchkov A.D., Sokolova L.S., Ayunov D.E., Permyakov M.E. Assesment of Potential of West Siberian Permafrost for the Carbon Dioxide Storage. Earth's Cryosphere, 2009, vol. 13, no 4, pp. 6268. 5. Gimaltdinov I.K., Khasanov M.K. Mathematical Model of the Formation of a Gas Hydrate on the Injection of Gas into a Stratum Partially Saturated with Ice. Journal of Applied Mathematics and Mechanics, 2016, vol. 80, no. 1, pp. 5764. DOI: 10.1016/j.jappmathmech.2016.05.009 6. Shagapov V.Sh., Rakova G.R., Khasanov M.K. On the Theory of Formation of Gas Hydrate in Partially Water-Saturated Porous Medium when Injecting Methane. High Temperature, 2016, vol. 54, no. 6, pp. 858866. DOI: 10.1134/S0018151X16060171 7. Shagapov V.Sh., Chiglintseva A.S., Belova S.V. On the Theory of Formation of a Gas Hydrate in a Heat-Insulated Space Compacted with Methane. Journal of Engineering Physics and Thermophysics, 2017, vol. 90, no. 5, pp. 11471161. DOI: 10.1007/s10891-017-1669-8 8. Shagapov V.Sh., Chiglintceva A.S., Belova S.V. Mathematical Modelling of Injection Gas Hydrate Formation into the Massif of Snow Saturated the Same Gas. Proceedings of the Mavlyutov Institute of Mechanics, 2016. vol. 11, no. 2, p. 233239. DOI: 10.21662/uim2016.2.034 9. Khasanov M.K. Injection of Liquid Hydrogen Sulde in a Layer Saturated with Oil and Water. Tyumen State University Herald. Physical and Mathematical Modeling. Oil, Gas, Energy, 2017, vol. 3, no. 2, pp. 7284. 10. Shagapov V.Sh., Khasanov M.K., Musakaev N.G., Ngoc Hai Duong. Theoretical Research of the Gas Hydrate Deposits Development Using the Injection of Carbon Dioxide. International Journal of Heat and Mass Transfer, 2017, vol. 107, pp. 347357. DOI: 10.1016/j.ijheatmasstransfer.2016.11.034 Received February 20, 2018 80 Bulletin of the South Ural State University. Ser. Mathematical Modelling, Programming & Computer Software (Bulletin SUSU MMCS), 2018, vol. 11, no. 2, pp. 7382 ÌÀÒÅÌÀÒÈ×ÅÑÊÎÅ ÌÎÄÅËÈÐÎÂÀÍÈÅ ÓÄÊ 519.6+532.546 DOI: 10.14529/mmp180206 ÌÀÒÅÌÀÒÈ×ÅÑÊÀß ÌÎÄÅËÜ ÎÁÐÀÇÎÂÀÍÈß ÃÀÇÎÃÈÄÐÀÒÀ ÑÅÐÎÂÎÄÎÐÎÄÀ ÏÐÈ ÅÃÎ ÈÍÆÅÊÖÈÈ Â ÏÐÈÐÎÄÍÛÉ ÏËÀÑÒ Ì.Ê. Õàñàíîâ1 , Ã.Ð. Ðàôèêîâà2 Ñòåðëèòàìàêñêèé ôèëèàë Áàøêèðñêîãî ãîñóäàðñòâåííîãî óíèâåðñèòåòà, ã. Ñòåðëèòàìàê, Ðîññèéñêàÿ Ôåäåðàöèÿ 2 Èíñòèòóò ìåõàíèêè èì. Ð.Ð. Ìàâëþòîâà îáîñîáëåííîå ñòðóêòóðíîå ïîäðàçäåëåíèå Óôèìñêîãî ôåäåðàëüíîãî èññëåäîâàòåëüñêîãî öåíòðà Ðîññèéñêîé àêàäåìèè íàóê, ã. Óôà, Ðîññèéñêàÿ Ôåäåðàöèÿ 1 Ïðåäñòàâëåíà ìàòåìàòè÷åñêàÿ ìîäåëü èíæåêöèè æèäêîãî ñåðîâîäîðîäà â ïîëóáåñêîíå÷íûé ïîðèñòûé ïëàñò, íàñûùåííûé íåôòüþ è âîäîé, ñîïðîâîæäàþùåéñÿ îáðàçîâàíèåì ãàçîãèäðàòà H2 S. Ðàññìîòðåí ñëó÷àé, êîãäà ãèäðàòîîáðàçîâàíèå ïðîèñõîäèò íà ôðîíòàëüíîé ãðàíèöå, à ôðîíò âûòåñíåíèÿ íåôòè ñåðîâîäîðîäîì îïåðåæàåò äàííóþ ãðàíèöó. Ìåòîäîì ñâåäåíèÿ ê àâòîìîäåëüíîé ïåðåìåííîé ïîñòðîåíû ðåøåíèÿ äëÿ äàâëåíèÿ è òåìïåðàòóðû â êàæäîé èç îáëàñòåé ïëàñòà. Çíà÷åíèÿ ïàðàìåòðîâ ïîäâèæíûõ ìåæôàçíûõ ãðàíèö íàéäåíû êàê ðåçóëüòàò èòåðàöèîííîé ïðîöåäóðû. Íà îñíîâå ïîëó÷åííûõ ðåøåíèé èññëåäîâàíà çàâèñèìîñòü êîîðäèíàò ìåæôàçíûõ ãðàíèö îò äàâëåíèÿ èíæåêöèè. Ïîêàçàíî, ÷òî äëÿ ñóùåñòâîâàíèÿ ðåøåíèé ñ äâóìÿ ðàçëè÷íûìè ìåæôàçíûìè ãðàíèöàìè âåëè÷èíà äàâëåíèÿ èíæåêöèè äîëæíà áûòü âûøå íåêîòîðîãî ïðåäåëüíîãî çíà÷åíèÿ. Ïîñòðîåíà çàâèñèìîñòü ïðåäåëüíîãî çíà÷åíèÿ äàâëåíèÿ èíæåêöèè îò íà÷àëüíîé òåìïåðàòóðû ïëàñòà ïðè ðàçíûõ çíà÷åíèÿõ òåìïåðàòóðû èíæåêòèðóåìîãî ñåðîâîäîðîäà. Ðåçóëüòàòû ðàñ÷åòîâ ïîêàçàëè, ÷òî ïîñòðîåííàÿ ìàòåìàòè÷åñêàÿ ìîäåëü ñ òðåìÿ îáëàñòÿìè â ïëàñòå äàåò àäåêâàòíîå îïèñàíèå ïðîöåññà ïðè âûñîêèõ çíà÷åíèÿõ äàâëåíèÿ èíæåêöèè, òåìïåðàòóðû çàêà÷èâàåìîãî ñåðîâîäîðîäà è íà÷àëüíîé òåìïåðàòóðû ïëàñòà. Êëþ÷åâûå ñëîâà: ìàòåìàòè÷åñêàÿ ìîäåëü; àâòîìîäåëüíîå ðåøåíèå; ïîðèñòàÿ ñðåäà; ôèëüòðàöèÿ; ãàçîãèäðàòû; ñåðîâîäîðîä. Ðàáîòà âûïîëíåíà ïðè ôèíàíñîâîé ïîääåðæêå ÐÔÔÈ è Ðåñïóáëèêè Áàøêîðòîñòàí (ïðîåêò 17-48-020123 ð− à). Ëèòåðàòóðà 1. Machel, H.G. Geological and Hydrogeological Evaluation of the Nisku Q-Pool in Alberta, Canada, for H2 S and/or CO2 Storage / H.G. Machel // Oil and Gas Science and Technology. 2005. V. 60. P. 5165. 2. Xu, T. Numerical Modeling of Injection and Mineral Trapping of CO2 with H2 S and SO2 in a Sandstone Formation / T. Xu, J.A. Apps, K. Pruess, H. Yamamoto // Chemical Geology. 2007. V. 24, 34. P. 319346. 3. Áûê, Ñ.Ø. Ãàçîâûå ãèäðàòû / Ñ.Ø. Áûê, Þ.Ô. Ìàêîãîí, Â.È. Ôîìèíà. Ì.: Õèìèÿ, 1980. 4. Äó÷êîâ, À.Ä. Îöåíêà âîçìîæíîñòè çàõîðîíåíèÿ óãëåêèñëîãî ãàçà â êðèîëèòîçîíå Çàïàäíîé Ñèáèðè / À.Ä. Äó÷êîâ, Ë.Ñ. Ñîêîëîâà, Ä.Å. Àþíîâ, Ì.Å. Ïåðìÿêîâ // Êðèîñôåðà Çåìëè. 2009. Ò. 13, 4. Ñ. 6268. 5. Ãèìàëòäèíîâ, È.Ê. Ìàòåìàòè÷åñêàÿ ìîäåëü îáðàçîâàíèÿ ãàçîãèäðàòà ïðè èíæåêöèè ãàçà â ïëàñò, ÷àñòè÷íî íàñûùåííûé ëüäîì / È.Ê. Ãèìàëòäèíîâ, Ì.Ê. Õàñàíîâ // Ïðèêëàäíàÿ ìàòåìàòèêà è ìåõàíèêà. 2016. Ò. 80, 1. Ñ. 8090. Âåñòíèê ÞÓðÃÓ. Ñåðèÿ ≪Ìàòåìàòè÷åñêîå ìîäåëèðîâàíèå è ïðîãðàììèðîâàíèå≫ (Âåñòíèê ÞÓðÃÓ ÌÌÏ). 2018. Ò. 11, 2. Ñ. 7382 81 M.K. Khasanov, G.R. Rakova 6. Øàãàïîâ, Â.Ø. Ê òåîðèè îáðàçîâàíèÿ ãàçîãèäðàòà â ÷àñòè÷íî âîäîíàñûùåííîé ïîðèñòîé ñðåäå ïðè íàãíåòàíèè ìåòàíà / Â.Ø. Øàãàïîâ, Ã.Ð. Ðàôèêîâà, Ì.Ê. Õàñàíîâ // Òåïëîôèçèêà âûñîêèõ òåìïåðàòóð. 2016. Ò. 54, 6. Ñ. 911920. 7. Øàãàïîâ, Â.Ø. Ê òåîðèè ïðîöåññà îáðàçîâàíèÿ ãàçîãèäðàòà â çàìêíóòîì òåïëîèçîëèðîâàííîì îáúåìå, îïðåññîâàííîì ìåòàíîì / Â.Ø. Øàãàïîâ, À.Ñ. ×èãëèíöåâà, Ñ.Â. Áåëîâà // Èíæåíåðíî-ôèçè÷åñêèé æóðíàë. 2017. Ò. 90, 5. Ñ. 12081222. 8. Øàãàïîâ, Â.Ø. Ìàòåìàòè÷åñêîå ìîäåëèðîâàíèå íàãíåòàíèÿ ãèäðàòîîáðàçóþùåãî ãàçà â ñíåæíûé ìàññèâ, íàñûùåííûé òåì æå ãàçîì / Â.Ø. Øàãàïîâ, À.Ñ. ×èãëèíöåâà, Ñ.Â. Áåëîâà // Òðóäû Èíñòèòóòà ìåõàíèêè èì. Ð.Ð. Ìàâëþòîâà Óôèìñêîãî íàó÷íîãî öåíòðà ÐÀÍ. 2016. Ò. 11, 2. Ñ. 233239. 9. Õàñàíîâ, Ì.Ê. Èíæåêöèÿ æèäêîãî ñåðîâîäîðîäà â ïëàñò, íàñûùåííûé íåôòüþ è âîäîé / Ì.Ê. Õàñàíîâ // Âåñòíèê Òþìåíñêîãî ãîñóäàðñòâåííîãî óíèâåðñèòåòà. Ôèçèêîìàòåìàòè÷åñêîå ìîäåëèðîâàíèå. Íåôòü, ãàç, ýíåðãåòèêà. 2017. Ò. 3, 2. Ñ. 7284. 10. Shagapov, V.Sh. Theoretical Research of the Gas Hydrate Deposits Development Using the Injection of Carbon Dioxide / V.Sh. Shagapov, M.K. Khasanov, N.G. Musakaev, Ngoc Hai Duong // International Journal of Heat and Mass Transfer. 2017. V. 107. P. 347357. Ìàðàò Êàìèëîâè÷ Õàñàíîâ, êàíäèäàò ôèçèêî-ìàòåìàòè÷åñêèõ íàóê, äîöåíò, êàôåäðà ≪Ïðèêëàäíàÿ èíôîðìàòèêà è ïðîãðàììèðîâàíèå≫, Ñòåðëèòàìàêñêèé ôèëèàë Áàøêèðñêîãî ãîñóäàðñòâåííîãî óíèâåðñèòåòà (ã. Ñòåðëèòàìàê, Ðîññèéñêàÿ Ôåäåðàöèÿ), hasanovmk@mail.ru. Ãóçàëü Ðèíàòîâíà Ðàôèêîâà, êàíäèäàò ôèçèêî-ìàòåìàòè÷åñêèõ íàóê, ó÷åíûé ñåêðåòàðü, Èíñòèòóò ìåõàíèêè èì. Ð.Ð. Ìàâëþòîâà îáîñîáëåííîå ñòðóêòóðíîå ïîäðàçäåëåíèå Óôèìñêîãî ôåäåðàëüíîãî èññëåäîâàòåëüñêîãî öåíòðà Ðîññèéñêîé àêàäåìèè íàóê (ã. Óôà, Ðîññèéñêàÿ Ôåäåðàöèÿ), rakova− guzal@mail.ru. Ïîñòóïèëà â ðåäàêöèþ 20 ôåâðàëÿ 2018 ã. 82 Bulletin of the South Ural State University. Ser. Mathematical Modelling, Programming & Computer Software (Bulletin SUSU MMCS), 2018, vol. 11, no. 2, pp. 7382