Динамика кристаллизации и транспорт магм Ключевского вулкана
реклама
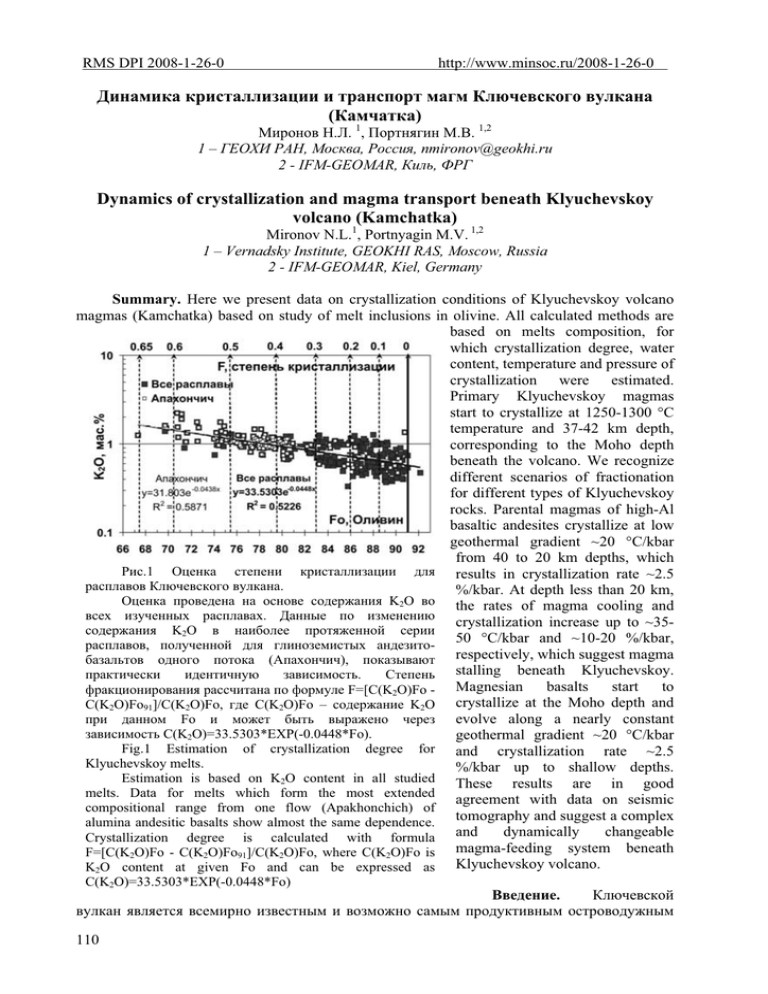
RMS DPI 2008-1-26-0 http://www.minsoc.ru/2008-1-26-0 Ⱦɢɧɚɦɢɤɚ ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ ɢ ɬɪɚɧɫɩɨɪɬ ɦɚɝɦ Ʉɥɸɱɟɜɫɤɨɝɨ ɜɭɥɤɚɧɚ (Ʉɚɦɱɚɬɤɚ) Ɇɢɪɨɧɨɜ ɇ.Ʌ. 1, ɉɨɪɬɧɹɝɢɧ Ɇ.ȼ. 1,2 1 – ȽȿɈɏɂ ɊȺɇ, Ɇɨɫɤɜɚ, Ɋɨɫɫɢɹ, [email protected] 2 - IFM-GEOMAR, Ʉɢɥɶ, ɎɊȽ Dynamics of crystallization and magma transport beneath Klyuchevskoy volcano (Kamchatka) Mironov N.L.1, Portnyagin M.V. 1,2 1 – Vernadsky Institute, GEOKHI RAS, Moscow, Russia 2 - IFM-GEOMAR, Kiel, Germany Summary. Here we present data on crystallization conditions of Klyuchevskoy volcano magmas (Kamchatka) based on study of melt inclusions in olivine. All calculated methods are based on melts composition, for which crystallization degree, water content, temperature and pressure of crystallization were estimated. Primary Klyuchevskoy magmas start to crystallize at 1250-1300 °C temperature and 37-42 km depth, corresponding to the Moho depth beneath the volcano. We recognize different scenarios of fractionation for different types of Klyuchevskoy rocks. Parental magmas of high-Al basaltic andesites crystallize at low geothermal gradient ~20 °C/kbar from 40 to 20 km depths, which Ɋɢɫ.1 Ɉɰɟɧɤɚ ɫɬɟɩɟɧɢ ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ ɞɥɹ results in crystallization rate ~2.5 ɪɚɫɩɥɚɜɨɜ Ʉɥɸɱɟɜɫɤɨɝɨ ɜɭɥɤɚɧɚ. %/kbar. At depth less than 20 km, Ɉɰɟɧɤɚ ɩɪɨɜɟɞɟɧɚ ɧɚ ɨɫɧɨɜɟ ɫɨɞɟɪɠɚɧɢɹ K2O ɜɨ the rates of magma cooling and ɜɫɟɯ ɢɡɭɱɟɧɧɵɯ ɪɚɫɩɥɚɜɚɯ. Ⱦɚɧɧɵɟ ɩɨ ɢɡɦɟɧɟɧɢɸ crystallization increase up to ~35ɫɨɞɟɪɠɚɧɢɹ K2O ɜ ɧɚɢɛɨɥɟɟ ɩɪɨɬɹɠɟɧɧɨɣ ɫɟɪɢɢ 50 °C/kbar and ~10-20 %/kbar, ɪɚɫɩɥɚɜɨɜ, ɩɨɥɭɱɟɧɧɨɣ ɞɥɹ ɝɥɢɧɨɡɟɦɢɫɬɵɯ ɚɧɞɟɡɢɬɨɛɚɡɚɥɶɬɨɜ ɨɞɧɨɝɨ ɩɨɬɨɤɚ (Ⱥɩɚɯɨɧɱɢɱ), ɩɨɤɚɡɵɜɚɸɬ respectively, which suggest magma ɩɪɚɤɬɢɱɟɫɤɢ ɢɞɟɧɬɢɱɧɭɸ ɡɚɜɢɫɢɦɨɫɬɶ. ɋɬɟɩɟɧɶ stalling beneath Klyuchevskoy. basalts start to ɮɪɚɤɰɢɨɧɢɪɨɜɚɧɢɹ ɪɚɫɫɱɢɬɚɧɚ ɩɨ ɮɨɪɦɭɥɟ F=[C(K2O)Fo - Magnesian crystallize at the Moho depth and C(K2O)Fo91]/C(K2O)Fo, ɝɞɟ C(K2O)Fo – ɫɨɞɟɪɠɚɧɢɟ K2O ɩɪɢ ɞɚɧɧɨɦ Fo ɢ ɦɨɠɟɬ ɛɵɬɶ ɜɵɪɚɠɟɧɨ ɱɟɪɟɡ evolve along a nearly constant ɡɚɜɢɫɢɦɨɫɬɶ C(K2O)=33.5303*EXP(-0.0448*Fo). geothermal gradient ~20 °C/kbar Fig.1 Estimation of crystallization degree for and crystallization rate ~2.5 Klyuchevskoy melts. %/kbar up to shallow depths. Estimation is based on K2O content in all studied These results are in good melts. Data for melts which form the most extended agreement with data on seismic compositional range from one flow (Apakhonchich) of tomography and suggest a complex alumina andesitic basalts show almost the same dependence. dynamically changeable Crystallization degree is calculated with formula and magma-feeding system beneath F=[C(K2O)Fo - C(K2O)Fo91]/C(K2O)Fo, where C(K2O)Fo is K2O content at given Fo and can be expressed as Klyuchevskoy volcano. C(K2O)=33.5303*EXP(-0.0448*Fo) ȼɜɟɞɟɧɢɟ. Ʉɥɸɱɟɜɫɤɨɣ ɜɭɥɤɚɧ ɹɜɥɹɟɬɫɹ ɜɫɟɦɢɪɧɨ ɢɡɜɟɫɬɧɵɦ ɢ ɜɨɡɦɨɠɧɨ ɫɚɦɵɦ ɩɪɨɞɭɤɬɢɜɧɵɦ ɨɫɬɪɨɜɨɞɭɠɧɵɦ 110 ɜɭɥɤɚɧɨɦ ɧɚ Ɂɟɦɥɟ. ɇɚɦɢ ɩɪɟɞɫɬɚɜɥɟɧɵ ɪɟɡɭɥɶɬɚɬɵ ɪɚɛɨɬɵ, ɧɚɩɪɚɜɥɟɧɧɨɣ ɧɚ ɨɰɟɧɤɭ ɭɫɥɨɜɢɣ ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ ɜ ɩɢɬɚɸɳɟɣ ɦɚɝɦɚɬɢɱɟɫɤɨɣ ɫɢɫɬɟɦɟ ɜɭɥɤɚɧɚ. ɋ ɷɬɨɣ ɰɟɥɶɸ ɛɵɥɨ ɢɡɭɱɟɧɨ ɛɨɥɟɟ 400 ɪɚɫɩɥɚɜɧɵɯ ɜɤɥɸɱɟɧɢɣ ɜ ɨɥɢɜɢɧɟ Fo91-67 ɢɡ ɪɚɡɥɢɱɧɨɝɨ ɬɢɩɚ ɩɨɪɨɞ ɨɬ ɜɵɫɨɤɨɦɚɝɧɟɡɢɚɥɶɧɵɯ ɛɚɡɚɥɶɬɨɜ ɞɨ ɜɵɫɨɤɨɝɥɢɧɨɡɟɦɢɫɬɵɯ ɚɧɞɟɡɢɬɨɛɚɡɚɥɶɬɨɜ, ɩɪɟɞɫɬɚɜɥɹɸɳɢɯ ɜɫɟ ɝɥɚɜɧɵɟ ɬɢɩɵ ɩɨɪɨɞ ɜɭɥɤɚɧɚ ɜ ɬɟɱɟɧɢɟ ɝɨɥɨɰɟɧɚ. Ɋɚɫɩɥɚɜɧɵɟ ɢ ɮɥɸɢɞɧɵɟ ɜɤɥɸɱɟɧɢɹ. Ɉɤɨɥɨ ɩɨɥɨɜɢɧɵ ɪɚɫɩɥɚɜɧɵɯ ɜɤɥɸɱɟɧɢɣ (197) ɛɵɥɢ ɩɪɟɞɫɬɚɜɥɟɧɵ ɪɚɫɤɪɢɫɬɚɥɥɢɡɨɜɚɧɧɵɦɢ ɪɚɡɧɨɫɬɹɦɢ, ɫ ɤɨɬɨɪɵɦɢ ɩɪɨɜɨɞɢɥɫɹ ɢɧɞɢɜɢɞɭɚɥɶɧɵɣ ɬɟɪɦɨɦɟɬɪɢɱɟɫɤɢɣ ɷɤɫɩɟɪɢɦɟɧɬ ɩɨ ɢɯ ɝɨɦɨɝɟɧɢɡɚɰɢɢ. ȼɬɨɪɚɹ ɩɨɥɨɜɢɧɚ (210) ɛɵɥɚ ɩɪɟɞɫɬɚɜɥɟɧɚ ɩɪɢɪɨɞɧɨɡɚɤɚɥɟɧɧɵɦɢ ɫɬɟɤɥɨɜɚɬɵɦɢ ɜɤɥɸɱɟɧɢɹɦɢ. ȼɫɟ ɫɬɟɤɥɚ ɜɤɥɸɱɟɧɢɣ ɛɵɥɢ ɩɪɨɚɧɚɥɢɡɢɪɨɜɚɧɵ ɧɚ ɫɨɞɟɪɠɚɧɢɟ ɩɟɬɪɨɝɟɧɧɵɯ ɷɥɟɦɟɧɬɨɜ, ɫɟɪɵ ɢ ɯɥɨɪɚ ɫ ɩɨɦɨɳɶɸ ɷɥɟɤɬɪɨɧɧɨɝɨ ɦɢɤɪɨɡɨɧɞɚ, ɢ ɛɨɥɟɟ ɱɟɬɜɟɪɬɢ ɜɫɟɯ ɫɬɟɤɨɥ - ɧɚ ɫɨɞɟɪɠɚɧɢɟ ɷɥɟɦɟɧɬɨɜ-ɩɪɢɦɟɫɟɣ, ɮɬɨɪɚ ɢ ɜɨɞɵ ɫ ɩɨɦɨɳɶɸ ɢɨɧɧɨɝɨ ɡɨɧɞɚ. ɋɨɫɬɚɜ ɦɢɧɟɪɚɥɚ-ɯɨɡɹɢɧɚ ɨɩɪɟɞɟɥɹɥɫɹ ɧɚ ɷɥɟɤɬɪɨɧɧɨɦ ɦɢɤɪɨɡɨɧɞɟ. Ⱦɥɹ ɩɨɥɭɱɟɧɢɹ ɫɨɫɬɚɜɚ ɪɚɫɩɥɚɜɨɜ ɫɨɫɬɚɜɵ ɫɬɟɤɨɥ ɜɤɥɸɱɟɧɢɣ ɛɵɥɢ ɫɤɨɪɪɟɤɬɢɪɨɜɚɧɵ ɞɨ ɪɚɜɧɨɜɟɫɢɹ ɫ ɨɥɢɜɢɧɨɦ-ɯɨɡɹɢɧɨɦ. Ʉɪɨɦɟ ɪɚɫɩɥɚɜɧɵɯ ɜɤɥɸɱɟɧɢɣ ɛɵɥɨ ɢɡɭɱɟɧɨ ɨɤɨɥɨ 20 ɡɟɪɟɧ ɨɥɢɜɢɧɚ ɢ ɤɥɢɧɨɩɢɪɨɤɫɟɧɚ, ɫɨɞɟɪɠɚɳɢɯ ɮɥɸɢɞɧɵɟ ɜɤɥɸɱɟɧɢɹ CO2. ɂɡɭɱɟɧɢɟ ɩɪɨɜɨɞɢɥɨɫɶ ɧɚ ɤɪɢɨɦɟɬɪɢɱɟɫɤɨɣ ɭɫɬɚɧɨɜɤɟ ɜ ȽȿɈɏɂ ɊȺɇ ɩɪɢ ɫɨɞɟɣɫɬɜɢɢ ȼ.Ȼ. ɇɚɭɦɨɜɚ. ɉɨɥɭɱɟɧɧɵɟ ɞɚɧɧɵɟ ɨ ɩɥɨɬɧɨɫɬɢ ɮɥɸɢɞɧɵɯ ɜɤɥɸɱɟɧɢɣ ɩɨɡɜɨɥɢɥɢ ɫɞɟɥɚɬɶ ɧɟɡɚɜɢɫɢɦɭɸ ɨɰɟɧɤɭ ɞɚɜɥɟɧɢɹ ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ. Ɇɟɬɨɞɵ ɨɰɟɧɤɢ ɭɫɥɨɜɢɣ ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ. ȼ ɨɫɧɨɜɟ ɦɟɬɨɞɨɜ ɨɰɟɧɤɢ ɭɫɥɨɜɢɣ ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ ɥɟɠɚɬ ɫɨɫɬɚɜɵ ɪɚɫɩɥɚɜɨɜ, ɩɨɥɭɱɟɧɧɵɟ ɩɨ ɫɨɫɬɚɜɭ ɪɚɫɩɥɚɜɧɵɯ ɜɤɥɸɱɟɧɢɣ ɜ ɨɥɢɜɢɧɟ. Ɉɰɟɧɟɧɵ ɫɬɟɩɟɧɶ ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ ɪɚɫɩɥɚɜɨɜ, ɫɨɞɟɪɠɚɧɢɟ ɜɨɞɵ, ɬɟɦɩɟɪɚɬɭɪɚ ɢ ɞɚɜɥɟɧɢɟ ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ. ɋɬɟɩɟɧɶ ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ (F) ɩɨɥɭɱɟɧɚ ɧɚ ɨɫɧɨɜɟ ɨɛɳɟɣ ɡɚɜɢɫɢɦɨɫɬɢ ɫɨɞɟɪɠɚɧɢɹ ɤɚɥɢɹ (K2O) ɜ ɪɚɫɩɥɚɜɚɯ ɨɬ ɫɨɫɬɚɜɚ ɨɥɢɜɢɧɚ-ɯɨɡɹɢɧɚ (Fo) (Ɋɢɫ. 1). ɋɨɞɟɪɠɚɧɢɟ ɜɨɞɵ ɞɥɹ ɪɚɫɩɥɚɜɨɜ, ɪɚɜɧɨɜɟɫɧɵɯ ɫ ɨɥɢɜɢɧɨɦ Fo>82, ɨɰɟɧɟɧɨ ɧɚ ɨɫɧɨɜɟ ɡɚɜɢɫɢɦɨɫɬɢ H2O=188.66*EXP(-0.0448*Fo), ɩɨɥɭɱɟɧɧɨɣ ɩɨ ɞɚɧɧɵɦ ɨ ɦɚɤɫɢɦɚɥɶɧɨ ɢɡɦɟɪɟɧɧɵɯ ɤɨɧɰɟɧɬɪɚɰɢɹɯ ɜɨɞɵ ɜ ɪɚɫɩɥɚɜɧɵɯ ɜɤɥɸɱɟɧɢɹɯ ɜ ɨɥɢɜɢɧɟ Fo91-82 ɫ ɭɱɟɬɨɦ ɧɚɤɨɩɥɟɧɢɹ ɜɨɞɵ ɩɪɢ ɮɪɚɤɰɢɨɧɢɪɨɜɚɧɢɢ. ɋɨɞɟɪɠɚɧɢɟ ɜɨɞɵ ɜ ɪɚɫɩɥɚɜɚɯ, ɪɚɜɧɨɜɟɫɧɵɯ ɫ ɛɨɥɟɟ ɠɟɥɟɡɢɫɬɵɦ ɨɥɢɜɢɧɨɦ, ɩɨɥɭɱɟɧɨ ɪɚɫɱɟɬɧɵɦ ɦɟɬɨɞɨɦ ɩɨ ɪɚɡɧɢɰɟ ɬɟɦɩɟɪɚɬɭɪ ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ ɨɥɢɜɢɧɚ ɢ ɩɥɚɝɢɨɤɥɚɡɚ (Danyushevsky et al., 1996), ɩɨɤɚɡɵɜɚɸɳɢɦ ɯɨɪɨɲɟɟ ɫɨɨɬɜɟɬɫɬɜɢɟ ɫ ɪɟɚɥɶɧɨ ɢɡɦɟɪɟɧɧɵɦɢ ɫɨɞɟɪɠɚɧɢɹɦɢ ɜɨɞɵ ɜɨ ɜɤɥɸɱɟɧɢɹɯ ɜ ɨɥɢɜɢɧɟ Fo82-67. Ɂɧɚɱɟɧɢɹ ɬɟɦɩɟɪɚɬɭɪɵ ɨɬɜɟɱɚɸɬ ɪɚɜɧɨɜɟɫɢɸ ɨɥɢɜɢɧ-ɪɚɫɩɥɚɜ (Ford et al., 1983) ɫ ɭɱɟɬɨɦ ɜɥɢɹɧɢɹ ɜɨɞɵ (Tɫɭɯɚɹ–TH2O=39.69*(H2O)0.73 – Ⱥlmeev et al., 2007) ɢ ɞɚɜɥɟɧɢɹ ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ (5 °ɋ/ɤɛɚɪ). Ɉɫɧɨɜɧɵɦ ɦɟɬɨɞɨɦ ɨɰɟɧɤɢ ɞɚɜɥɟɧɢɹ ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ ɞɥɹ ɪɚɜɧɨɜɟɫɢɹ Ol-Cpx-ɪɚɫɩɥɚɜ ɛɵɥ ɦɟɬɨɞ ɢɡɥɨɠɟɧɧɵɣ ɜ (Danyushevsky et al., 1996), ɤɨɬɨɪɵɣ ɛɵɥ ɩɪɨɜɟɪɟɧ ɧɚ ɷɤɫɩɟɪɢɦɟɧɬɚɥɶɧɵɯ ɫɨɫɬɚɜɚɯ ɫ ɪɚɡɥɢɱɧɵɦ ɫɨɞɟɪɠɚɧɢɟɦ ɜɨɞɵ (ɩɨ ɞɚɧɧɵɦ ɪɚɛɨɬ 1993-2007 ɝɝ.) Ɍɚɤɠɟ ɞɥɹ ɪɚɜɧɨɜɟɫɢɹ Cpx-ɪɚɫɩɥɚɜ ɢɫɩɨɥɶɡɨɜɚɥɫɹ ɦɟɬɨɞ (Putirka et al., 2003). ȼ ɤɚɱɟɫɬɜɟ ɧɟɡɚɜɢɫɢɦɨɣ ɨɰɟɧɤɢ ɢɫɩɨɥɶɡɨɜɚɥɢɫɶ ɡɧɚɱɟɧɢɹ, ɩɨɥɭɱɟɧɧɵɟ ɩɨ ɮɥɸɢɞɧɵɦ ɜɤɥɸɱɟɧɢɹɦ CO2. Ƚɥɭɛɢɧɵ ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ ɨɰɟɧɢɜɚɥɢɫɶ ɜ ɫɨɨɬɜɟɬɫɬɜɢɢ ɫ ɫɨɨɬɧɨɲɟɧɢɟɦ 1 ɤɛɚɪ ~ 3.5 ɤɦ. Ɍɚɛɥɢɰɚ 1. ɋɪɟɞɧɢɟ ɫɨɫɬɚɜɵ ɢ ɨɰɟɧɟɧɧɵɟ ɭɫɥɨɜɢɹ ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ ɞɥɹ ɜɵɫɨɤɨɦɚɝɧɟɡɢɚɥɶɧɨɝɨ (1) ɢ ɜɵɫɨɤɨɝɥɢɧɨɡɟɦɢɫɬɨɝɨ (2) ɪɚɫɩɥɚɜɨɜ Ʉɥɸɱɟɜɫɤɨɝɨ ɜɭɥɤɚɧɚ. Ɋɚɫɩɥɚɜ Fo #Mg SiO2 TiO2 Al2O3 FeO* MnO MgO CaO Na2O K2O P2O5 ɋɭɦɦɚ 1, N=24 90.4 70.9 48.05 0.8 14.94 8.51 0.08 11.6 12.5 2.64 0.63 0.13 99.9 std 0.3 1.0 1.16 0.08 0.78 0.01 0.03 0.56 0.96 0.23 0.2 0.04 2, N=75 78.9 47.9 51.55 1.13 18.95 9.25 0.16 4.78 9.01 3.76 1.01 0.21 99.8 std 1.0 1.9 1.54 0.10 0.78 0.63 0.05 0.37 0.62 0.34 0.14 0.02 Ɋɚɫɩɥɚɜ 1, N=24 std 2, N=75 std F, % 0.02 0.01 0.42 0.03 H2O-1 3.3 0.0 5.5 0.3 H2O-2 H2O-3 2.3 4.4 0.8 3.8 T-1, C 1298 15 1123 13 T-2, C 1270 24 1032 29 P-1, ɤɛ 13.4 1.9 5.0 1.8 P-2, ɤɛ 2.9 0.5 n=4 P-3, ɤɛ 9.3 0.6 n=3 4.7 0.9 n=2 111 ɉɪɢɦɟɱɚɧɢɟ. 1 – ɫɪɟɞɧɢɣ ɫɨɫɬɚɜ ɪɚɫɩɥɚɜɨɜ, ɪɚɜɧɨɜɟɫɧɵɯ ɫ ɧɚɢɛɨɥɟɟ ɦɚɝɧɟɡɢɚɥɶɧɵɦɢ ɨɥɢɜɢɧɚɦɢ (Fo90-91), 2 – ɫɪɟɞɧɢɣ ɫɨɫɬɚɜ, ɨɬɜɟɱɚɸɳɢɣ ɪɚɫɩɥɚɜɚɦ, ɪɚɜɧɨɜɟɫɧɵɯ ɫ ɨɥɢɜɢɧɨɦ Fo77-81 ɢ ɫɨɞɟɪɠɚɧɢɟɦ Al2O3>17 ɦɚɫ.%. H2O-1 – ɡɧɚɱɟɧɢɹ ɜɨɞɵ ɜ ɦɚɫ. %, ɪɚɫɫɱɢɬɚɧɧɵɟ ɫ ɭɱɟɬɨɦ ɮɪɚɤɰɢɨɧɢɪɨɜɚɧɢɹ ɩɪɢ ɢɫɯɨɞɧɨɦ ɫɨɞɟɪɠɚɧɢɢ ɜɨɞɵ 3.2 ɦɚɫ.%. H2O-2 – ɫɨɞɟɪɠɚɧɢɟ ɜɨɞɵ, ɪɚɫɫɱɢɬɚɧɧɨɟ ɩɨ ɪɚɡɧɢɰɟ ɬɟɦɩɟɪɚɬɭɪ ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ Ol ɢ Pl (Danyushevsky et al., 1996). H2O-3 – ɢɡɦɟɪɟɧɧɵɟ ɦɚɤɫɢɦɚɥɶɧɵɟ ɤɨɧɰɟɧɬɪɚɰɢɢ ɜɨɞɵ ɦɟɬɨɞɨɦ ɜɬɨɪɢɱɧɨ-ɢɨɧɧɨɣ ɦɚɫɫ ɫɩɟɤɬɪɨɦɟɬɪɢɢ (ɢɨɧɧɵɣ ɡɨɧɞ). T-1 – ɪɚɫɱɟɬɧɵɟ ɡɧɚɱɟɧɢɹ ɬɟɦɩɟɪɚɬɭɪɵ ɜ °C, ɩɪɢ ɫɭɯɢɯ ɭɫɥɨɜɢɹɯ ɢ ɞɚɜɥɟɧɢɢ 1 ɚɬɦ., T-2 – ɡɧɚɱɟɧɢɹ ɬɟɦɩɟɪɚɬɭɪɵ ɫ ɭɱɟɬɨɦ ɫɨɞɟɪɠɚɧɢɹ ɜɨɞɵ ɢ ɞɚɜɥɟɧɢɹ (P-1). P-1 – ɪɚɫɱɟɬɧɵɟ ɡɧɚɱɟɧɢɹ ɞɚɜɥɟɧɢɹ ɜ ɤɛɚɪ ɞɥɹ ɪɚɜɧɨɜɟɫɢɹ OlCpx-ɪɚɫɩɥɚɜ. P-2 – ɞɚɜɥɟɧɢɟ, ɪɚɫɫɱɢɬɚɧɧɨɟ ɩɨ ɩɥɨɬɧɨɫɬɢ ɜɤɥɸɱɟɧɢɣ CO2 ɜ ɨɥɢɜɢɧɟ ɩɪɢ T 1030 °ɋ. P-3 – ɞɚɜɥɟɧɢɟ ɞɥɹ ɪɚɜɧɨɜɟɫɢɹ Cpx-ɪɚɫɩɥɚɜ - ɞɥɹ ɪɚɫɩɥɚɜɚ (1) ɨɬɜɟɱɚɟɬ ɞɚɜɥɟɧɢɸ ɞɥɹ ɦɚɝɧɟɡɢɚɥɶɧɨɝɨ Cpx Ɋɢɫ.2 Ɋɚɡɥɢɱɧɵɟ ɪɟɠɢɦɵ ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ ɩɪɢ ɧɚɢɛɨɥɟɟ ɨɛɪɚɡɨɜɚɧɢɢ ɦɚɝɧɟɡɢɚɥɶɧɵɯ ɛɚɡɚɥɶɬɨɜ ɢ ɝɥɢɧɨɡɟɦɢɫɬɵɯ (#Mg91), ɜɫɬɪɟɱɟɧɧɨɝɨ ɜ ɜɢɞɟ ɜɤɪɚɩɥɟɧɧɢɤɨɜ ɜ ɪɚɜɧɨɜɟɫɢɢ ɫ ɚɧɞɟɡɢɬɨɛɚɡɚɥɶɬɨɜ Ʉɥɸɱɟɜɫɤɨɝɨ ɜɭɥɤɚɧɚ. ɦɚɝɧɟɡɢɚɥɶɧɵɦɢ 1 – ɫɪɟɞɧɢɟ ɡɧɚɱɟɧɢɹ ɞɚɜɥɟɧɢɹ ɢ ɫɬɟɩɟɧɢ ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ ɧɚɢɛɨɥɟɟ ɞɥɹ ɪɚɫɩɥɚɜɨɜ ɦɚɝɧɟɡɢɚɥɶɧɵɯ ɛɚɡɚɥɶɬɨɜ, ɭɫɪɟɞɧɟɧɧɵɯ ɪɚɫɩɥɚɜɚɦɢ (Fo91, ɫɪɟɞɧɢɣ Fo90-90.5, ɱɟɪɟɡ 1 ɧɨɦɟɪ ɩɨ Fo. 2 - ɫɪɟɞɧɢɟ ɡɧɚɱɟɧɢɹ ɞɥɹ ɪɚɫɩɥɚɜɨɜ ɫɪɟɞɧɢɣ Fo90-91). Ⱦɥɹ ɪɚɫɩɥɚɜɚ (2) ɞɚɜɥɟɧɢɸ ɞɥɹ ɝɥɢɧɨɡɟɦɢɫɬɵɯ ɚɧɞɟɡɢɬɨɛɚɡɚɥɶɬɨɜ. 3 – ɫɪɟɞɧɢɣ ɫɨɫɬɚɜ ɨɬɜɟɱɚɟɬ ɜɵɫɨɤɨɝɥɢɧɨɡɟɦɢɫɬɵɯ ɪɚɫɩɥɚɜɨɜ (Al2O3>17 ɦɚɫ.%, Fo82- ɤɪɢɫɬɚɥɥɢɱɟɫɤɢɯ ɜɤɥɸɱɟɧɢɣ Cpx ɜ ɨɥɢɜɢɧɟ ɢ ɪɚɫɩɥɚɜɨɜ, ɪɚɜɧɨɜɟɫɧɵɯ 77). 4 ɢ 5 – ɨɰɟɧɤɚ ɞɚɜɥɟɧɢɹ ɩɨ ɮɥɸɢɞɧɵɦ ɜɤɥɸɱɟɧɢɹɦ CO2 ɜ ɨɥɢɜɢɧɟ ɦɚɝɧɟɡɢɚɥɶɧɵɯ ɛɚɡɚɥɶɬɨɜ (4) ɢ ɨɥɢɜɢɧɟ ɢ ɫ ɨɥɢɜɢɧɨɦ Fo78-79. Ⱦɚɜɥɟɧɢɟ ɤɥɢɧɨɩɢɪɨɤɫɟɧɟ ɝɥɢɧɨɡɟɦɢɫɬɵɯ ɚɧɞɟɡɢɬɨ-ɛɚɡɚɥɶɬɨɜ (5). ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ ɩɟɪɜɢɱɧɵɯ ɦɚɝɦ Ⱦɥɹ 1 ɢ 2 ɩɨɤɚɡɚɧɵ ɞɚɧɧɵɟ ɞɥɹ ɪɚɫɩɥɚɜɨɜ ɫɨ ɫɬɟɩɟɧɶɸ ɩɨɥɭɱɟɧɨ ɫ ɭɱɟɬɨɦ ɡɧɚɱɟɧɢɣ, ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ F>0.5 (Fo>75). ȼɟɪɬɢɤɚɥɶɧɵɟ ɥɢɧɢɢ ɩɨɥɭɱɟɧɧɵɯ ɞɥɹ ɪɚɜɧɨɜɟɫɢɣ Olɩɨɤɚɡɵɜɚɸɬ ɜɟɥɢɱɢɧɭ ɫɬɚɧɞɚɪɬɧɵɯ ɨɬɤɥɨɧɟɧɢɣ. Cpx-ɪɚɫɩɥɚɜ ɢ Cpx-ɪɚɫɩɥɚɜ, ɢ ɇɚɤɥɨɧɧɵɦɢ ɥɢɧɢɹɦɢ ɩɨɤɚɡɚɧɵ ɪɚɡɥɢɱɧɵɟ ɡɧɚɱɟɧɢɹ ɦɨɠɟɬ ɛɵɬɶ ɨɰɟɧɟɧɨ ɤɚɤ 10.5-12 ɫɤɨɪɨɫɬɢ ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ ɩɪɢ ɩɨɞɴɟɦɟ ɦɚɝɦ. Ƚɥɭɛɢɧɵ ɜ ɤɛɚɪ. Fo ɨɥɢɜɢɧɚ-ɯɨɡɹɢɧɚ. FeO* ɤɦ ɧɚɧɟɫɟɧɵ ɫɨɝɥɚɫɧɨ 1 ɤɛɚɪ ~3.5 ɤɦ. Ɉɰɟɧɤɢ ɩɨ FeO ɨɛɳɟɟ. Std – ɡɧɚɱɟɧɢɹ ɫɬɚɧɞɚɪɬɧɵɯ ɨɬɤɥɨɧɟɧɢɣ ɨɬ ɞɚɜɥɟɧɢɸ ɫɞɟɥɚɧɵ ɩɨ ɦɟɬɨɞɭ (Danyushevsky et al., 1996). Fig.2 Different crystallization scenarios for magnesian basalts ɫɪɟɞɧɟɝɨ. and alumina andesitic basalts of Klyuchevskoy volcano. 1 – average values of pressure and crystallization degree for ɋɪɟɞɧɢɟ ɫɨɫɬɚɜɵ parental melts of magnesian basalts, averaged by 1 number of ɜɵɫɨɤɨɦɚɝɧɟɡɢɚɥɶɧɨɝɨ ɢ Fo. 2 – average values for parental melts of alumina basaltic ɜɵɫɨɤɨɝɥɢɧɨɡɟɦɢɫɬɨɝɨ andesites. 3 – average composition of high-Al melts ɪɚɫɩɥɚɜɨɜ ɢ ɭɫɪɟɞɧɟɧɧɵɟ (Al2O3>17 ɦɚɫ.%, Fo81-77). 4 and 5 – pressure estimates for ɡɧɚɱɟɧɢɹ ɭɫɥɨɜɢɣ ɢɯ CO2 fluid inclusions in olivine from magnesian basalts (4), ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ ɩɪɢɜɟɞɟɧɵ ɜ and in Ol and Cpx from alumina andesitic basalts (5). For 1 ɬɚɛɥ. 1. and 2 data for melts with F>0.5 (Fo>75) are shown. Vertical ɍɫɥɨɜɢɹ ɨɛɪɚɡɨɜɚɧɢɹ ɢ lines show standard deviations. Different crystallization rates at magma ascent are shown by inclined lines. Depths in km ɪɟɠɢɦɵ ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ ɦɚɝɦ ɜɭɥɤɚɧɚ. are according to relation: 1 kb~3.5 km. Pressure was Ʉɥɸɱɟɜɫɤɨɝɨ ɉɟɪɜɢɱɧɵɟ ɜɵɫɨɤɨestimated using method as in (Danyushevsky et al., 1996). ɦɚɝɧɟɡɢɚɥɶɧɵɟ ɛɚɡɚɥɶɬɨɜɵɟ ɦɚɝɦɵ Ʉɥɸɱɟɜɫɤɨɝɨ ɜɭɥɤɚɧɚ ɧɚɱɢɧɚɸɬ ɤɪɢɫɬɚɥɥɢɡɨɜɚɬɶɫɹ ɩɪɢ ɬɟɦɩɟɪɚɬɭɪɟ 1250-1300 °C, ɫɨɞɟɪɠɚɧɢɢ ɜɨɞɵ ~3 ɦɚɫ. % ɢ ɞɚɜɥɟɧɢɢ 10.5-12 ɤɛɚɪ, ɨɬɜɟɱɚɸɳɟɦ ɝɥɭɛɢɧɟ (37-42 ɤɦ) 112 ɝɪɚɧɢɰɵ Ɇɨɯɨ ɩɨɞ ɜɭɥɤɚɧɨɦ (Ɍɚɛɥ. 1). ɉɨɫɥɟɞɭɸɳɚɹ ɮɪɚɤɰɢɨɧɧɚɹ ɤɪɢɫɬɚɥɥɢɡɚɰɢɹ ɜɫɟɯ ɬɢɩɨɜ ɦɚɝɦ ɩɪɨɢɫɯɨɞɢɬ ɜ ɭɫɥɨɜɢɹɯ ɞɟɤɨɦɩɪɟɫɫɢɢ, ɯɨɬɹ ɫɤɨɪɨɫɬɢ ɨɫɬɵɜɚɧɢɹ ɢ ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ ɡɧɚɱɢɬɟɥɶɧɨ ɜɚɪɶɢɪɭɸɬ ɞɥɹ ɪɚɡɥɢɱɧɵɯ ɩɨɪɰɢɣ ɦɚɝɦ. ɉɨɥɭɱɟɧɧɵɟ ɞɚɧɧɵɟ ɦɨɝɭɬ ɫɜɢɞɟɬɟɥɶɫɬɜɨɜɚɬɶ ɨ ɞɜɭɯ ɪɚɡɥɢɱɧɵɯ ɫɰɟɧɚɪɢɹɯ ɩɨɞɴɟɦɚ ɢ ɷɜɨɥɸɰɢɢ ɦɚɝɦ ɩɨɞ ɜɭɥɤɚɧɨɦ (Ɋɢɫ. 2). Ɉɛɪɚɡɨɜɚɧɢɟ ɜɵɫɨɤɨɝɥɢɧɨɡɟɦɢɫɬɵɯ ɚɧɞɟɡɢɬɨ-ɛɚɡɚɥɶɬɨɜ, ɹɜɥɹɸɳɢɯɫɹ ɩɪɟɨɛɥɚɞɚɸɳɢɦ ɬɢɩɨɦ ɥɚɜ Ʉɥɸɱɟɜɫɤɨɝɨ ɜɭɥɤɚɧɚ, ɩɪɨɢɫɯɨɞɢɬ ɜ 2 ɫɬɚɞɢɢ. ɇɚ ɩɟɪɜɨɣ ɫɬɚɞɢɢ (ɧɚ ɝɥɭɛɢɧɟ ~ ɨɬ 40 ɞɨ 20 ɤɦ) ɢɯ ɪɨɞɨɧɚɱɚɥɶɧɵɟ ɦɚɝɦɵ ɤɪɢɫɬɚɥɥɢɡɭɸɬɫɹ ɩɪɢ ɝɟɨɬɟɪɦɢɱɟɫɤɨɦ ɝɪɚɞɢɟɧɬɟ ~20 °C/ɤɛɚɪ ɢ ɫɤɨɪɨɫɬɢ ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ ~2.5 %/ɤɛɚɪ. Ⱦɚɥɟɟ ɧɚ ɝɥɭɛɢɧɚɯ ɦɟɧɟɟ 20 ɤɦ (ɞɚɜɥɟɧɢɢ ~ < 6 ɤɛɚɪ) ɫɤɨɪɨɫɬɢ ɨɫɬɵɜɚɧɢɹ ɢ ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ ɦɚɝɦ ɭɜɟɥɢɱɢɜɚɸɬɫɹ ɞɨ ~35-50 °C/ɤɛɚɪ ɢ ~10-20 %/ɤɛɚɪ ɫɨɨɬɜɟɬɫɬɜɟɧɧɨ, ɱɬɨ ɝɨɜɨɪɢɬ ɨ ɡɚɦɟɞɥɟɧɢɢ ɩɨɞɴɟɦɚ ɦɚɝɦ ɢ ɢɯ ɧɚɤɨɩɥɟɧɢɢ ɩɨɞ ɜɭɥɤɚɧɨɦ. ȼɵɫɨɤɨɝɥɢɧɨɡɟɦɢɫɬɵɟ ɪɚɫɩɥɚɜɵ, ɛɥɢɡɤɢɟ ɤ ɫɨɫɬɚɜɭ ɢɡɜɟɪɠɟɧɧɵɯ ɦɚɝɦ ɨɛɪɚɡɭɸɬɫɹ ɩɪɢ ɞɚɜɥɟɧɢɢ 5-3 ɤɛɚɪ (18 - 10 ɤɦ) ɢ ɬɟɦɩɟɪɚɬɭɪɟ 1030±30°C ɢ ɫɨɞɟɪɠɚɧɢɢ ɜɨɞɵ ~ 4.5 ɦɚɫ.% (Ɋɢɫ. 2, Ɍɚɛɥɢɰɚ 1). Ɇɚɝɧɟɡɢɚɥɶɧɵɟ ɛɚɡɚɥɶɬɵ, ɢɡɜɟɪɠɟɧɢɹ ɤɨɬɨɪɵɯ ɩɪɨɢɫɯɨɞɢɥɢ ɢɡ ɩɨɛɨɱɧɵɯ ɤɨɧɭɫɨɜ ɧɚ ɩɨɞɧɨɠɢɢ ɜɭɥɤɚɧɚ, ɧɚɱɢɧɚɸɬ ɤɪɢɫɬɚɥɥɢɡɨɜɚɬɶɫɹ ɧɚ ɝɥɭɛɢɧɟ ɝɪɚɧɢɰɵ Ɇɨɯɨ ɢ ɷɜɨɥɸɰɢɨɧɢɪɭɸɬ ɞɨ ɦɟɧɶɲɢɯ ɝɥɭɛɢɧ ɫ ɩɨɫɬɨɹɧɧɵɦɢ ɝɟɨɬɟɪɦɢɱɟɫɤɢɦ ɝɪɚɞɢɟɧɬɨɦ ~20 °C/ɤɛɚɪ ɢ ɫɤɨɪɨɫɬɶɸ ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ ~2.5 %/ɤɛɚɪ. ɇɟɡɧɚɱɢɬɟɥɶɧɚɹ ɫɬɚɬɢɫɬɢɤɚ ɩɨ ɫɨɫɬɚɜɭ ɪɚɫɩɥɚɜɨɜ, ɯɚɪɚɤɬɟɪɢɡɭɸɳɢɯ ɡɚɤɥɸɱɢɬɟɥɶɧɵɟ ɫɬɚɞɢɢ ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ ɦɚɝɧɟɡɢɚɥɶɧɵɯ ɛɚɡɚɥɶɬɨɜ ɧɟ ɩɨɡɜɨɥɹɟɬ ɭɜɟɪɟɧɧɨ ɝɨɜɨɪɢɬɶ ɨ ɝɥɭɛɢɧɟ ɢɯ ɤɪɢɫɬɚɥɥɢɡɚɰɢɢ, ɬɟɦ ɧɟ ɦɟɧɟɟ, ɩɨɥɭɱɟɧɧɵɟ ɞɚɧɧɵɟ ɩɨɡɜɨɥɹɸɬ ɩɪɟɞɩɨɥɚɝɚɬɶ, ɱɬɨ ɜɵɫɨɤɨɦɚɝɧɟɡɢɚɥɶɧɵɟ ɦɚɝɦɵ ɦɨɝɭɬ ɤɪɢɫɬɚɥɥɢɡɨɜɚɬɶɫɹ ɜ ɨɬɞɟɥɶɧɵɯ ɤɚɧɚɥɚɯ, ɜ ɨɛɯɨɞ ɨɫɧɨɜɧɨɣ ɩɢɬɚɸɳɟɣ ɫɢɫɬɟɦɵ ɜɭɥɤɚɧɚ, ɧɚɱɢɧɚɹ ɭɠɟ ɫ ɝɥɭɛɢɧɵ ~20 ɤɦ. ɉɨɥɭɱɟɧɧɵɟ ɪɟɡɭɥɶɬɚɬɵ ɧɚɯɨɞɹɬɫɹ ɜ ɯɨɪɨɲɟɦ ɫɨɨɬɜɟɬɫɬɜɢɢ ɫ ɞɚɧɧɵɦɢ ɫɟɣɫɦɢɱɟɫɤɨɣ ɬɨɦɨɝɪɚɮɢɢ ɢ ɩɪɟɞɩɨɥɚɝɚɸɬ ɤɨɦɩɥɟɤɫɧɭɸ ɩɢɬɚɸɳɭɸ ɦɚɝɦɚɬɢɱɟɫɤɭɸ ɫɢɫɬɟɦɭ ɩɨɞ Ʉɥɸɱɟɜɫɤɢɦ ɜɭɥɤɚɧɨɦ. ɗɬɚ ɫɢɫɬɟɦɚ ɩɨ ɜɢɞɢɦɨɦɭ ɱɭɜɫɬɜɢɬɟɥɶɧɚ ɤ ɢɡɦɟɧɟɧɢɸ ɪɟɠɢɦɚ ɩɨɫɬɭɩɥɟɧɢɹ ɦɚɝɦ ɢɡ ɦɚɧɬɢɢ, ɭɫɢɥɟɧɢɟ ɤɨɬɨɪɨɝɨ ɦɨɠɟɬ ɜɵɡɵɜɚɬɶ ɢɡɛɵɬɨɱɧɨɟ ɞɚɜɥɟɧɢɟ ɢ ɞɟɮɨɪɦɚɰɢɢ ɜ ɤɨɪɟ, ɨɛɟɫɩɟɱɢɜɚɹ ɧɨɜɵɟ ɩɭɬɢ ɞɥɹ ɩɨɞɴɟɦɚ ɦɚɝɦ ɤ ɩɨɜɟɪɯɧɨɫɬɢ. Ɋɚɛɨɬɚ ɜɵɩɨɥɧɟɧɚ ɩɪɢ ɩɨɞɞɟɪɠɤɟ ɊɎɎɂ (ɝɪɚɧɬ ʋ 07-05-00807ɚ). Ʌɢɬɟɪɚɬɭɪɚ: Almeev R.R., Holtz F., Koepke J., Parat F., Botcharnikov R.E. The effect of H2O on olivine crystallization in MORB: Experimental calibration at 200 MPa // American Mineralogist. 2007. V. 92. I. 4. P. 670-674. Danyushevsky L.V., Sobolev A.V., Dmitriev L.V. Estimation of the pressure of crystallization and H2O content of MORB and BABB glasses: calibration of an empirical technique // Mineralogy and Petrology. 1996. V. 57. P. 185-204. Ford C.E., Russel D.G., Graven J.A., Fisk M.R. Olivine-liquid equilibria: temperature, pressure and composition dependence of the crystal/liquid cation partition coefficients for Mg, Fe2+, Ca and Mn // Journal of Petrology. 1983. V. 24. P. 256-265. Putirka K., Mikaelian H., Ryerson F., Shaw H. New clinopyroxene-liquid thermobarometers for mafic, evolved, and volatile-bearing lava compositions, with applications to lavas from Tibet and the Snake River Plain, Idaho // American Mineralogist. 2003. V. 88. P. 1542–1554. 113
